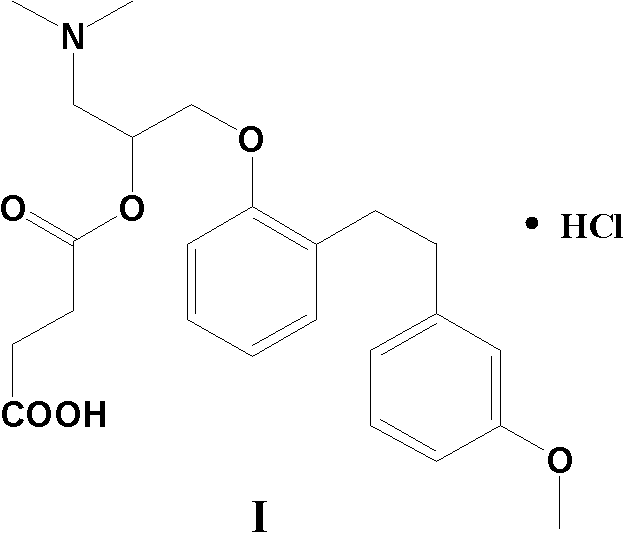
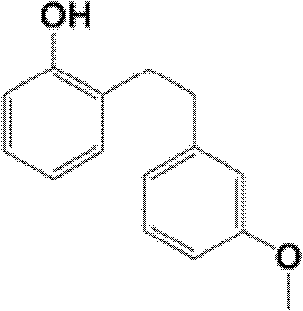
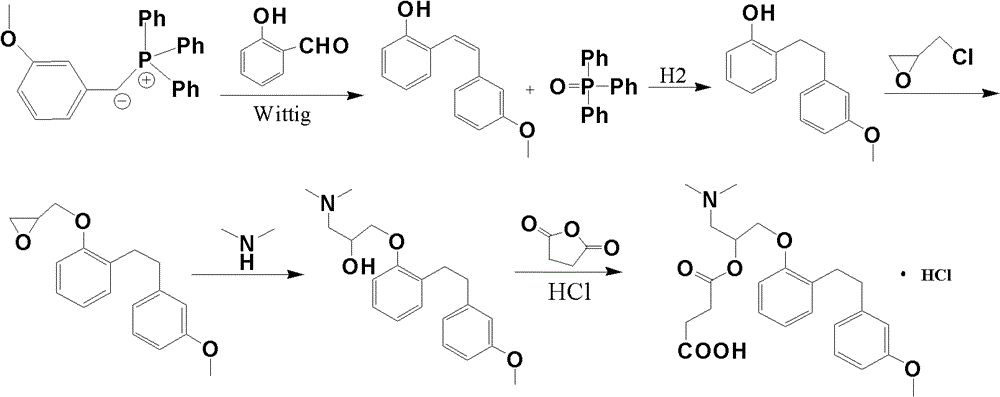
Preparation method of sarpogrelate hydrochloride
A technology of sarcogrelate hydrochloride and acidity, which is applied in the field of preparation of sarcogrelate hydrochloride, can solve the problems of inability to meet the needs of large-scale production, difficult sarcogrelate hydrochloride, high reaction temperature requirements, etc., and achieves low pollution, high purity, and raw materials low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1: the preparation of 3-methoxybenzyl alcohol (II):
[0076] Add 447.6g (3.29mol) of 3-methoxybenzaldehyde, 170ml of methanol and 2g of sodium hydroxide into the reaction flask, cool down to about 0°C in an ice bath, and add 34.3g of sodium borohydride powder in batches under mechanical stirring (0.91mol), control the rate of addition, keep the temperature below 50°C, after the addition is complete, place it at 25°C for 4 hours, TLC (ethyl ethyl ester: n-hexane = 1: 8) reacts completely, and then in an ice bath Next, add glacial acetic acid to the yellow reaction solution to adjust the pH value to 4 to 5; evaporate methanol under reduced pressure, add 300ml of water, separate the layers, and extract the water layer twice with 300ml of ethyl acetate. The combined organic layers were washed twice with 200 ml of saturated aqueous sodium carbonate solution, separated, dried over anhydrous sodium sulfate, filtered, and evaporated to remove the solvent under reduce...
Embodiment 2
[0077] Embodiment 2: Preparation of 3-methoxybenzyl chloride (III):
[0078] 440.6g (3.19mol) of intermediate II was added to a 1000ml reaction flask, the ice bath was lowered to 0°C, and 420ml of concentrated hydrochloric acid (37%) was added under vigorous stirring, and the reaction was completed at 30°C for 2 hours, then the temperature was raised to Reaction was carried out at 50°C for 7 hours, and TLC (ethyl ethyl ester:n-hexane=1:8) showed that the reaction was complete. Cool in an ice-water bath again to 0°C, add 400ml of water to the reaction solution, stir for 30 minutes, separate the layers, save the lower orange organic layer, extract the water layer twice with 350ml ethyl acetate, and save the organic layer with the orange separated from the previous step Combined, washed twice with 400ml saturated aqueous sodium bicarbonate solution, separated, dried over anhydrous sodium sulfate, filtered, evaporated to dryness under reduced pressure to obtain a light yellow liqu...
Embodiment 3
[0079] Embodiment 3: Preparation of 3-methoxybenzyl bromide (III):
[0080] Add 440.6g (3.19mol) of intermediate II to a 2000ml reaction flask, drop the ice bath to 0°C, add 700ml of hydrobromic acid (40%) under vigorous stirring, complete the addition, react at 30°C for 2 hours, then raise the temperature Reaction was carried out at 45°C for 6 hours, and TLC (ethyl ethyl ester:n-hexane=1:8) showed that the reaction was complete. Cool in an ice-water bath again to 0°C, add 400ml of water to the reaction solution, stir for 30 minutes, separate the layers, save the lower orange organic layer, extract the water layer twice with 350ml ethyl acetate, and save the organic layer with the orange separated from the previous step Combined, washed twice with 400ml of saturated aqueous sodium bicarbonate solution, separated, dried over anhydrous sodium sulfate, stored in the dark, filtered, and evaporated to dryness under reduced pressure to obtain a pale yellow liquid, decompressed by an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com