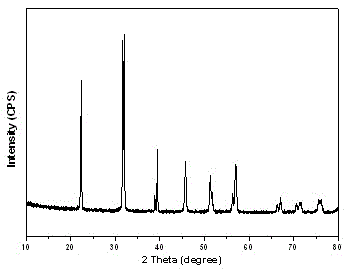
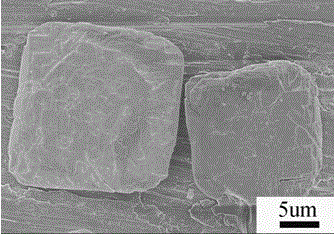
Preparation method of bismuth ferrite BiFeO3 monocrystal micrometer sheet
A technology of micron flakes and bismuth ferrite, which is applied in the direction of single crystal growth, single crystal growth, chemical instruments and methods, etc., can solve the problems of low product purity, difficulty in controlling particle size and shape, poor controllability of reaction, etc., and achieve The process is simple, the feasibility and controllability are good, and the cost is low
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0021] 1) Weigh ferric nitrate and bismuth ammonium citrate at a molar ratio of 1:1, add deionized water and stir thoroughly to form a suspension containing bismuth ammonium citrate and ferric nitrate;
[0022] 2) Under stirring, add an aqueous potassium hydroxide solution to the suspension containing bismuth ammonium citrate and ferric nitrate prepared in step 1), to obtain a suspension containing bismuth and iron oxyhydroxide precipitates;
[0023] 3) Transfer the obtained suspension of oxyhydroxide precipitation containing bismuth and iron to the inner tank of the reactor, adjust the volume of the reaction material in the inner tank of the reactor with deionized water to reach 90% of the volume of the inner tank of the reactor, and stir In 10 minutes, in the reaction mass, the molar concentration of ferric nitrate and bismuth amine citrate was 0.05mol / L, and the molar concentration of potassium hydroxide was 0.6 mol / L, and the volume base of molar concentration was all intro...
example 2
[0026] 1) Weigh ferric nitrate and bismuth ammonium citrate at a molar ratio of 1:1, add deionized water and stir thoroughly to form a suspension containing bismuth ammonium citrate and ferric nitrate;
[0027] 2) Under stirring, add an aqueous potassium hydroxide solution to the suspension containing bismuth ammonium citrate and ferric nitrate prepared in step 1), to obtain a suspension containing bismuth and iron oxyhydroxide precipitates;
[0028] 3) Transfer the obtained suspension of oxyhydroxide precipitation containing bismuth and iron to the inner tank of the reactor, adjust the volume of the reaction material in the inner tank of the reactor with deionized water to reach 90% of the volume of the inner tank of the reactor, and stir In 10 minutes, in the reaction mass, the molar concentration of ferric nitrate and bismuth amine citrate was 0.05mol / L, and the molar concentration of potassium hydroxide was 0.4 mol / L, and the volume base of molar concentration was all intro...
example 3
[0031] 1) Weigh ferric nitrate and bismuth ammonium citrate at a molar ratio of 1:1, add deionized water and stir thoroughly to form a suspension containing bismuth ammonium citrate and ferric nitrate;
[0032] 2) Under stirring, add an aqueous potassium hydroxide solution to the suspension containing bismuth ammonium citrate and ferric nitrate prepared in step 1), to obtain a suspension containing bismuth and iron oxyhydroxide precipitates;
[0033] 3) Transfer the obtained suspension of oxyhydroxide precipitation containing bismuth and iron to the inner tank of the reactor, adjust the volume of the reaction material in the inner tank of the reactor with deionized water to reach 90% of the volume of the inner tank of the reactor, and stir In 10 minutes, in the reaction mass, the molar concentration of ferric nitrate and bismuth ammonium citrate was 0.05mol / L, and the molar concentration of potassium hydroxide was 0.8 mol / L, and the volume base of molar concentration was all in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com