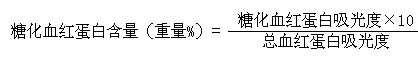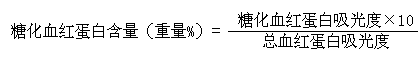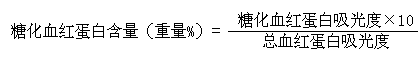Test method and test kit of glycosylated hemoglobin
A technology of glycosylated hemoglobin and a detection method, which is applied in the field of medical clinical biochemical diagnostic reagents, and achieves the effects of ensuring the sameness, being convenient to use, and shortening the operation time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1, a detection kit, including packaging box, ion resin, hemolytic agent, standard solution;
[0032] The ionic resin is 5-20mg / mld cation exchange resin suspension, which is Rex-70, and it is packaged as a single serving, and it is a disposable slit plastic cuvette with a cover or a disposable vial with a cover; the hemolytic agent is 0.1% saponin solution is used for hemolysis, and the standard solution is freeze-dried whole blood, which has been calibrated and assigned by the national standard, used for calibration, and reconstituted with distilled water before use.
[0033] The storage conditions and validity period are: the original reagent should be stored in the dark at 2-8°C, and the validity period is 18 months;
[0034] The above kit is used for in vitro quantitative determination of the concentration of glycosylated hemoglobin (Glycosylated hemoglobin A1, HbA1c) in human whole blood.
[0035] Applicable instruments are suitable for all kinds of equ...
Embodiment 2
[0053] Embodiment 2, a detection kit, including packing box, ion resin, hemolytic agent, standard solution;
[0054] The ion resin is a cation exchange resin suspension of 5 mg / mld, which is Rex-70, and it is packaged as a single serving, and it is a disposable slit plastic cuvette with a lid or a disposable vial with a lid, 3ml per serving Quantity; the hemolytic agent is 0.1% saponin solution, used for hemolysis, 0.5ml per person, the standard solution is freeze-dried whole blood, calibrated and assigned by the national standard, and reconstituted with distilled water before use.
[0055] The method for detecting glycosylated hemoglobin using the above-mentioned detection kit is carried out according to the following steps: the ion exchange resin homogeneous adsorption separation method is adopted, and the specific adsorption of the ion resin to hemoglobin is utilized. First, a hemolytic agent is used to prepare a hemoglobin sample. Under the condition of ~40°C, fully mix th...
Embodiment 3
[0070] Embodiment 3, a detection kit, including packaging box, ion resin, hemolytic agent, standard solution;
[0071] Ion resin is 10mg / mld cation exchange resin suspension, Rex-70, in single-serve packaging, in disposable slit plastic cuvettes with lids or in disposable vials with lids, 1.5ml per serving Filling volume: The hemolytic agent is 0.1% saponin solution, used for hemolysis, 0.5ml per person, the standard solution is freeze-dried whole blood, calibrated and assigned by the national standard, and reconstituted with distilled water before use.
[0072] The method for detecting glycosylated hemoglobin using the above-mentioned detection kit is carried out according to the following steps: the ion exchange resin homogeneous adsorption separation method is adopted, and the specific adsorption of the ion resin to hemoglobin is utilized. First, a hemolytic agent is used to prepare a hemoglobin sample. Under the condition of ~40°C, fully mix the ion resin with the prepared...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com