Method of using ketoxime ether for preparation of alkoxyl amine hydrochloride and alkoxyl amine hydrochloride preparation method
A technology of hydrocarbyloxyamine hydrochloride and ketoxime ether, which is applied in the field of preparing hydrocarbyloxyamine hydrochloride and hydrocarbyloxyamine hydrochloride from ketoxime ether, and can solve the operation danger, yield and low cost. problems such as low yield, to achieve the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] According to a second aspect of the present invention, the present invention also provides a method for preparing alkoxyamine hydrochloride, the method comprising the following steps:
[0032] (1) Under the reaction conditions of ketoammoxime reaction, in the presence of a titanium silicon molecular sieve catalyst, acetone or butanone, hydrogen peroxide and ammonia dissolved in an organic solvent are fed into the reactor for contact reaction to obtain acetone oxime or butanone oxime, wherein the molar ratio of hydrogen peroxide to acetone or butanone is 1-1.3;
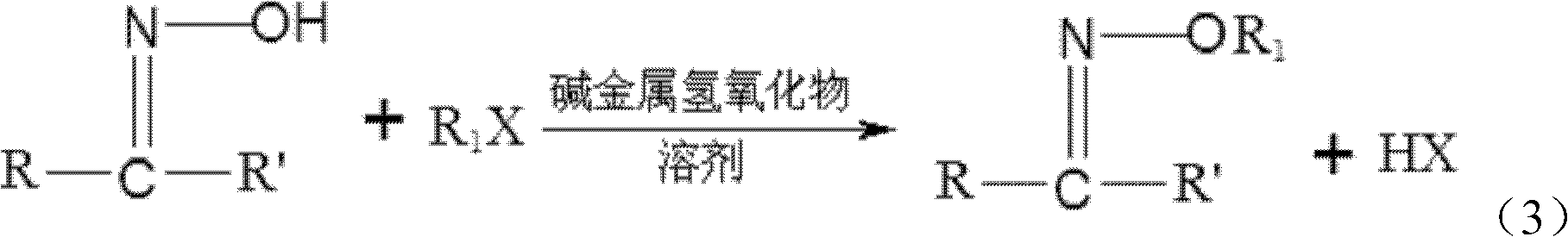
[0033] (2) Under the substitution reaction conditions, the acetone oxime or butanone oxime obtained in step (1) is contacted with a halogenated hydrocarbon, and the acetone oxime ether or butanone oxime ether is separated from the product obtained after the contact reaction ;
[0034] (3) According to the method for preparing alkoxyamine hydrochloride from ketoxime ether described above, the acetone oxime ether...
Embodiment approach
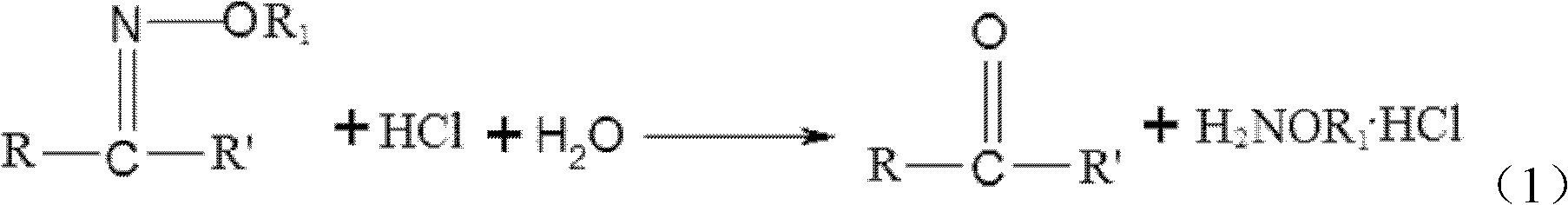
[0056] According to the method provided by the present invention, as can be seen from the above reaction formula (1), another product (product except alkoxyamine hydrochloride) of step (3) is acetone or butanone, and in In the catalytic rectification process, acetone or butanone is discharged from the top of the rectification tower; in addition, since there is no additional organic solvent in the step (3), the acetone or butanone in the overhead distillate passes through or not After concentration, it can be directly recycled for use in step (1), and the presence of a small amount of HCl in the overhead distillate also helps to stabilize hydrogen peroxide in step (1). Therefore, according to another preferred embodiment of the present invention, the method further includes: recycling the overhead distillate of the rectification tower to be used as at least part of the acetone or butane in step (1) after being concentrated or not. ketone. In this preferred embodiment, the acet...
Embodiment 1
[0061] This example is used to illustrate the method for preparing alkoxyamine hydrochloride from ketoxime ether of the present invention and.
[0062] (1) Preparation of acetone oxime
[0063] Add 1.2g of TS-1 molecular sieve catalyst (prepared according to the method of Example 1 in CN1301599A), 11.6g of acetone, 35g of tert-butanol and 22g of ammonia water with a concentration of 25% by weight into a 200ml reactor. The mixture in the reactor was fully mixed, the reactor was sealed, and the reaction temperature was controlled at 80°C. Continuously add 26 g of hydrogen peroxide with a concentration of 30% by weight with a micro feed pump. Hydrogen peroxide was continuously pumped in for 2 hours, and the reaction was continued for 1 hour. After the reaction, the solid catalyst was separated from the mixed liquid, and the resulting liquid was cooled to room temperature, extracted 3 times with tetrachloroethylene, and the extract layer liquid was combined, then subjected to va...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com