Magnetic particle chemiluminescence kit for detecting nitrofural metabolites, and applications thereof
A chemiluminescence reagent, the technology of nitrofurazone, which is applied to the magnetic particle chemiluminescence kit for detecting nitrofurazone metabolites and its application field, can solve the problems of poor reagent stability, reaction time and temperature influence, and achieve low detection time and fast detection , the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of the concrete component of embodiment one kit
[0035] 1) Synthesis of nitrofurazone metabolite hapten
[0036] The nitrofurazone metabolite is reacted with m-nitrobenzaldehyde to obtain a nitrofurazone metabolite derivative, and then a nitrofurazone metabolite hapten with -COOH is synthesized through a chemical reaction.
[0037] 2) Preparation of nitrofurazone metabolite artificial antigen
[0038] The immunogen was obtained by coupling the nitrofurazone metabolite hapten with bovine serum albumin (BSA) by the mixed anhydride method.
[0039] 3) Preparation of monoclonal antibodies
[0040] Animal immunization: Immunize Balb / c mice with the immunogen at a dose of 100 μg / mouse to produce antiserum.
[0041] Cell fusion and cloning: Splenocytes from immunized Balb / c mice were fused with SP2 / 0 myeloma cells at a ratio of 9:1 to obtain hybridoma cell lines of monoclonal antibodies.
[0042] Cell cryopreservation and recovery: the hybridoma cells were...
Embodiment 2
[0053] The formation of embodiment two kits
[0054] A magnetic particle direct competition chemiluminescent detection kit for detecting nitrofurazone metabolites was constructed so that it contained the following components:
[0055] Fluorescent marker of FITC-labeled nitrofurazone metabolite monoclonal antibody
[0056] Luminescent marker of ABEI-labeled nitrofurazone metabolite hapten
[0057] Separation reagent of paramagnetic nanobeads coated with goat anti-FITC monoclonal antibody
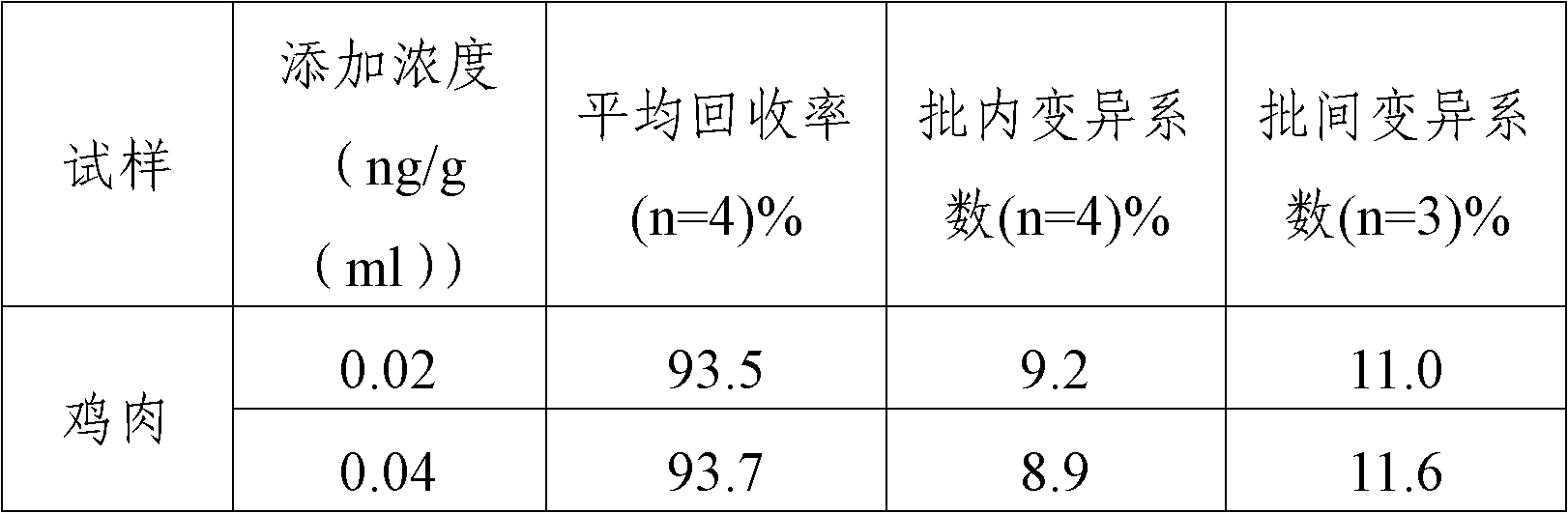
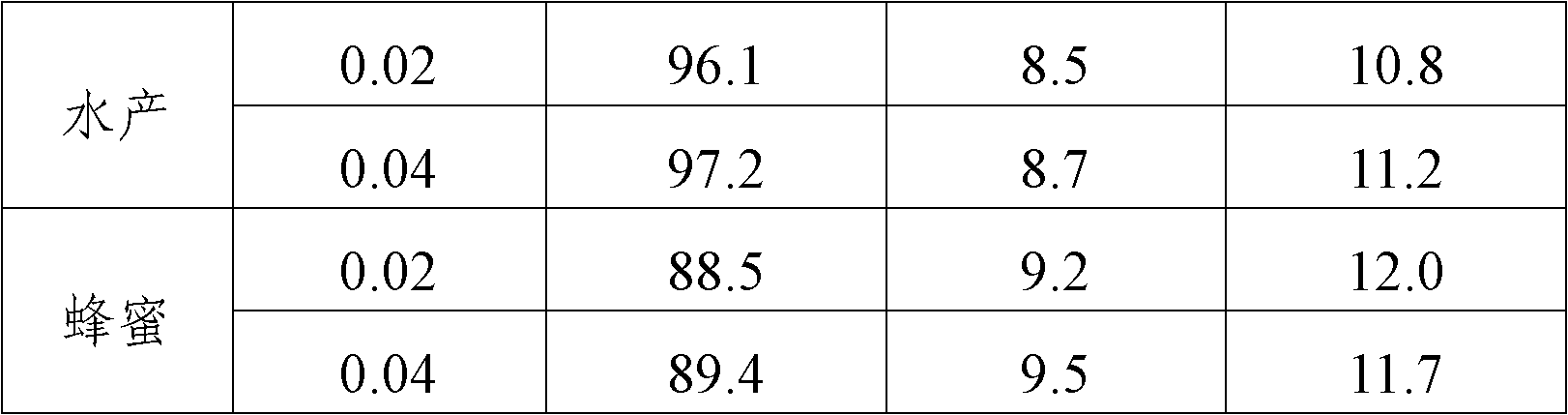
[0058] Furacilin metabolite standard solution (0ng / ml, 0.01ng / ml, 0.03ng / ml, 0.1ng / ml, 0.3ng / ml, 1.0ng / ml), the standard diluent is pH7.4, 0.03%NaN 3 , 0.05mol / L tris buffer. The percentage content is a mass percentage content.
[0059] The concentrations of nitrofurazone metabolite control solution are 0.02ng / ml and 0.5ng / ml respectively, and the diluent of quality control substance is pH7.4, 0.03%NaN 3 , 0.05mol / L tris buffer. The percentage content is a mass percentage content.
[0...
Embodiment 3
[0061] The detection of nitrofurazone metabolites in the actual sample of embodiment three
[0062] 1. Sample pretreatment
[0063] (1) Pretreatment methods for tissues such as muscle and liver
[0064] Use a homogenizer to homogenize the sample and take 1.0±0.05g of homogeneous material. Add 5ml of methanol-water solution, shake with a shaker for 5min, centrifuge at room temperature (20-25°C) for 10min at 3000g, remove all supernatant; (this step can reduce sample matrix interference), add 4ml of deionized water, and vortex Vortex to dissolve, add 0.5ml 1M hydrochloric acid solution and 100μl derivatization reagent, shake fully with a shaker; incubate overnight at 37°C (about 16h); add 5ml 0.1M dipotassium hydrogen phosphate solution, 0.4ml 1M sodium hydroxide respectively Solution and 10ml of ethyl acetate, shake vigorously with a shaker for 30s; centrifuge at room temperature (20-25°C) for 10min above 3000g; take 5ml of ethyl acetate phase into a 10ml dry glass test tube,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com