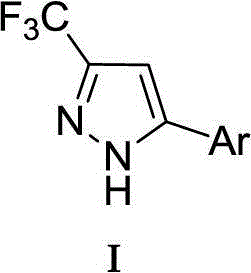
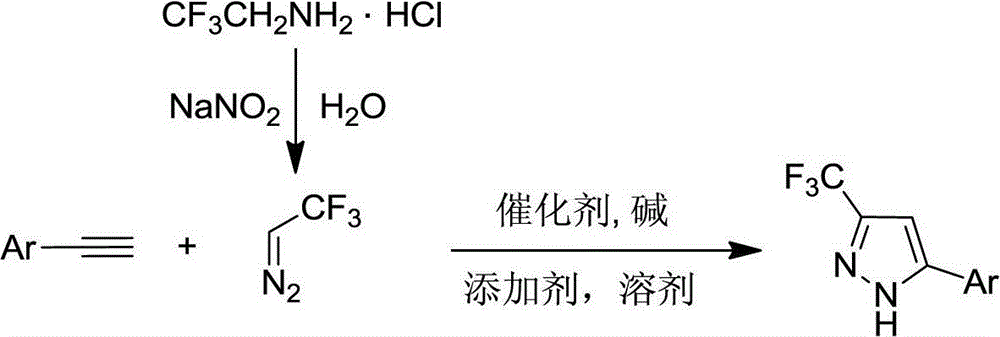
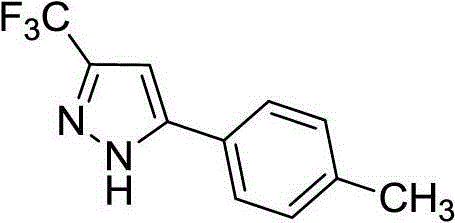
Preparation method of 5-aryl-3-trifluoromethyl-1H-pyrazole compound
A technology for trifluoromethyl compounds, which is applied in the field of pyrazole compounds and its preparation, can solve the problems of long reaction time, many steps, cumbersome operations, etc., and achieve high atom utilization, mild reaction conditions, and good substrate universality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0014] Example 1: Preparation of 5-(4-methylphenyl)-3-trifluoromethyl-1H-pyrazole
[0015]
[0016] Weigh Ag into a dry 50mL Schlenk reaction flask 2 O (0.46g, 2.0mmol), KOAc (0.20g, 2.0mmol), DABCO (0.011g, 0.1mmol), replace argon 3 times, add 4.0mL dry dichloromethane to the system, 2-(4- Methylphenyl) acetylene (0.12g, 1.0mmol), placed at room temperature and stirred for 2h, then introduced CF generated on site into the system 3 CHN 2 Gas (0.68g, 5.0mmol CF 3 CH 2 NH 2 Dissolve HCl in 20ml of water, add dropwise 0.41g, 6.0mmol NaNO 2 Solution prepared), ventilated for about 3h, ventilated, placed at room temperature and stirred for 20h. The reaction was complete as detected by TLC. Add 20 mL of ethyl acetate and 20 mL of water to the system, separate the layers, extract the aqueous phase with ethyl acetate (20 mL x 2), combine the organic phases and wash twice with water (40 mL), anhydrous MgSO 4 Drying, column chromatography (eluent: petroleum ether / ethyl acetate...
example 2
[0017] Example 2: Preparation of 5-(2-methoxyphenyl)-3-trifluoromethyl-1H-pyrazole
[0018]
[0019] A method similar to Experiment 1, using silver acetate as a catalyst, potassium carbonate as a base, 2-methoxyphenyl-1-acetylene, silver acetate, potassium carbonate, DABCO and 2,2,2-trifluoromethyldiazo The molar proportion of ethane is 1 / 3 / 3 / 1 / 10, reacts 24h at 50 ℃, synthesizes 5-(2-methoxyphenyl)-3-trifluoromethyl-1H-pyrazole, receives The rate is 72%. 1 H NMR (400MHz, CDCl 3 )δ4.01(s,3H),6.87(s,1H),7.05-7.10(m,2H),7.37(t,J=8.0Hz,1H),7.65(d,J=7.6Hz,1H), 11.82(s,1H); 13 C NMR (100MHz, CDCl 3 )δ156.0,143.0(q,J C-F =38.0Hz),142.1,130.2,128.0,121.6,121.5(q,J C-F =266.7Hz),116.3,111.7,100.9,55.8; 19 F NMR (376MHz, CDCl 3 )δ-62.11(s,3F).
example 3
[0020] Example 3: Preparation of 5-(3-methoxyphenyl)-3-trifluoromethyl-1H-pyrazole
[0021]
[0022] A method similar to Experiment 1, using silver oxide as a catalyst, potassium acetate as a base, chloroform as a solvent, 3-methoxyphenyl-1-acetylene, silver oxide, potassium acetate, DABCO and 2,2,2- The molar ratio of trifluoromethyldiazoethane is 1 / 2 / 2 / 0.1 / 5, reacted for 20h at 0°C to synthesize 5-(3-methoxyphenyl)-3-trifluoromethane Base-1H-pyrazole, yield 92%. 1 H NMR(400MHz,DMSO-d6)δ3.82(s,3H),6.95(d,J=7.2Hz,1H),7.18(s,1H),7.34-7.41(m,2H),7.44(s, 1H),14.08(s,1H); 13 C NMR (100MHz, DMSO-d6) δ159.8, 144.0, 142.2 (q, J C-F =40.0Hz),130.2,129.4,121.8(q,J C-F =265.8Hz),117.8,114.6,111.0,101.1,55.2; 19 F NMR (376 MHz, DMSO-d6) δ-60.70 (s, 3F).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com