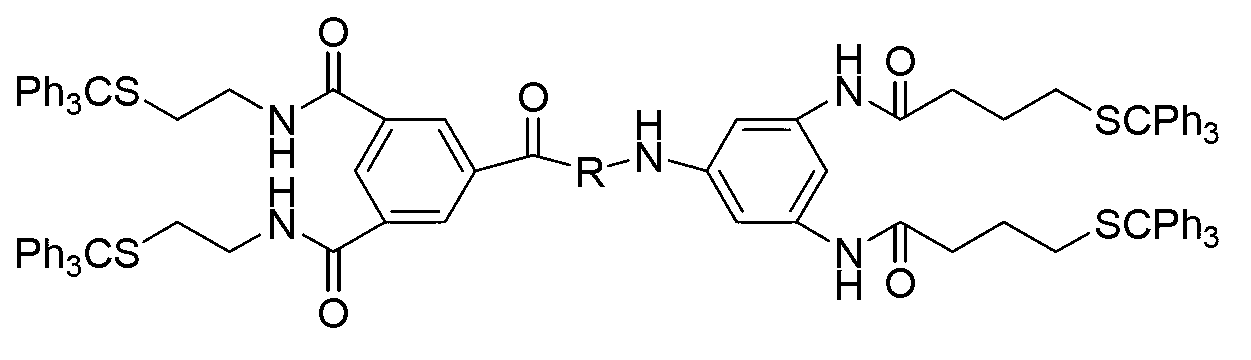
Module compound with hydrogen bond sequence specificity combination and preparation method thereof
A technology of modular compounds and compounds, applied in the preparation of sulfides, organic chemistry, etc., can solve the problems of long synthetic routes, difficult large-scale preparations, complex reaction processes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Embodiment 1, the synthesis of hydrophilic module P2
[0088] The hydrophilic module P2 is synthesized from compounds A4 and P1. The specific synthesis process is as follows:
[0089]
[0090] 1.1 Synthesis of intermediate compound A4
[0091] 1.1.1 Synthesis of reaction intermediate A2
[0092] Add 3,5-diaminobenzoic acid (1.52 g, 10 mmol), 5 mL of concentrated sulfuric acid, and 50 mL of methanol into a 100 mL single-necked round bottom flask, and reflux at 100° C. for 6-8 hours. Rotate the reaction solution under reduced pressure to remove methanol to a viscous liquid, pour it into a separatory funnel and add 300mL ethyl acetate to dilute, wash 3 times with 200mL tap water, wash 2 times with 150mL saturated sodium chloride solution, dichloromethane organic phase Dry with anhydrous sodium sulfate for 6h. Filtration and rotary evaporation under reduced pressure to remove ethyl acetate gave 1.21 g of reddish-brown solid, Yield: 72.89%.
[0093] 1 H NMR (400MHz...
Embodiment 2
[0132] Embodiment 2, the synthesis of hydrophobic module BLOCK-1
[0133] The hydrophobic module compound BLOCK-1 is synthesized from compounds B5 and Y3.
[0134] 2.1 Synthesis of intermediate compound Y3
[0135] The specific synthesis process is as follows:
[0136]
[0137] 2.1.1 Synthesis of intermediate compound Y1
[0138] Add 5-aminoisophthalic acid (5.02g, 28mmol) and 25mL of 4M aqueous sodium hydroxide solution into a 50mL single-necked round-bottomed flask, stir in an ice bath, add chloroacetyl chloride (8mL, 101mmol) dropwise, and a solid precipitates out. After 40 minutes, the dropwise addition was completed, and the reaction was continued for 30 minutes. Add dropwise 6M hydrochloric acid aqueous solution to acidify to pH ≈ 1.5, terminate the reaction, filter with suction, rinse with ice water for 300-400 mL, collect the filter cake and dry it in a vacuum oven to obtain 6.34 g of white solid, Yield 85.34%. Mp>250°C.
[0139] 1 H NMR(DMSO-d6,400MHz)δ13.3...
Embodiment 3
[0155] Embodiment 3, the synthesis of hydrophobic module BLOCK-2
[0156] The hydrophobic module compound BLOCK-2 is synthesized from compound B4 and Y4 or Y2. Wherein, compound Y4 is synthesized as follows:
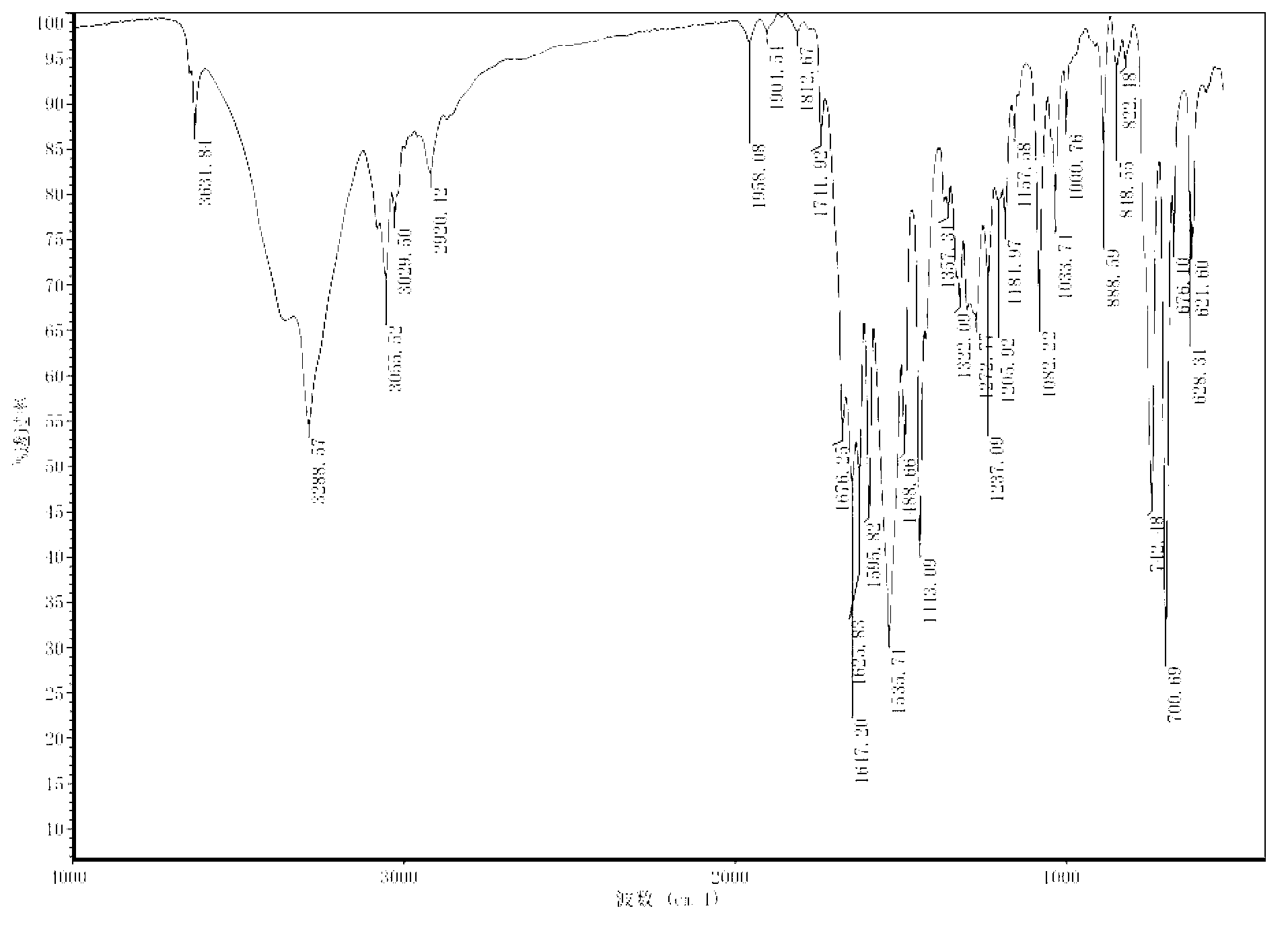
[0157] Add Y2 (2.15g, 2.5mmol), potassium formate (0.84g, 10mmol), tetra-n-butylamine (0.80g, 2.5mmol) 1mL tap water, DMF 20mL, and oil bath at 100°C for 12h in a 50mL single-necked round bottom flask. The reaction was also diluted with 100 mL of ethyl acetate, washed 4 times with 100 mL of tap water, washed 3 times with saturated brine, dried over anhydrous sodium sulfate, filtered, and the ethyl acetate was removed under reduced pressure to obtain a white powder Y41.78g, Yield: 84.76% . The infrared spectrum of Y4 is as follows Figure 5 shown.
[0158] 1 H NMR (CDCl 3,400MHz)δ8.94(b,1H,NH),7.93(s,2H,ArH),7.75(s,1H,ArH),7.09~7.42(m,30H,ArH),7.06(b,2H,NH ),4.06(s,2H,CH 2 ),3.23(m,4H,CH 2 ),2.48(m,4H,CH 2 ).IR(KBr,cm -1 ):3329,3287,3082,3055,3030,2980,2934,28...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com