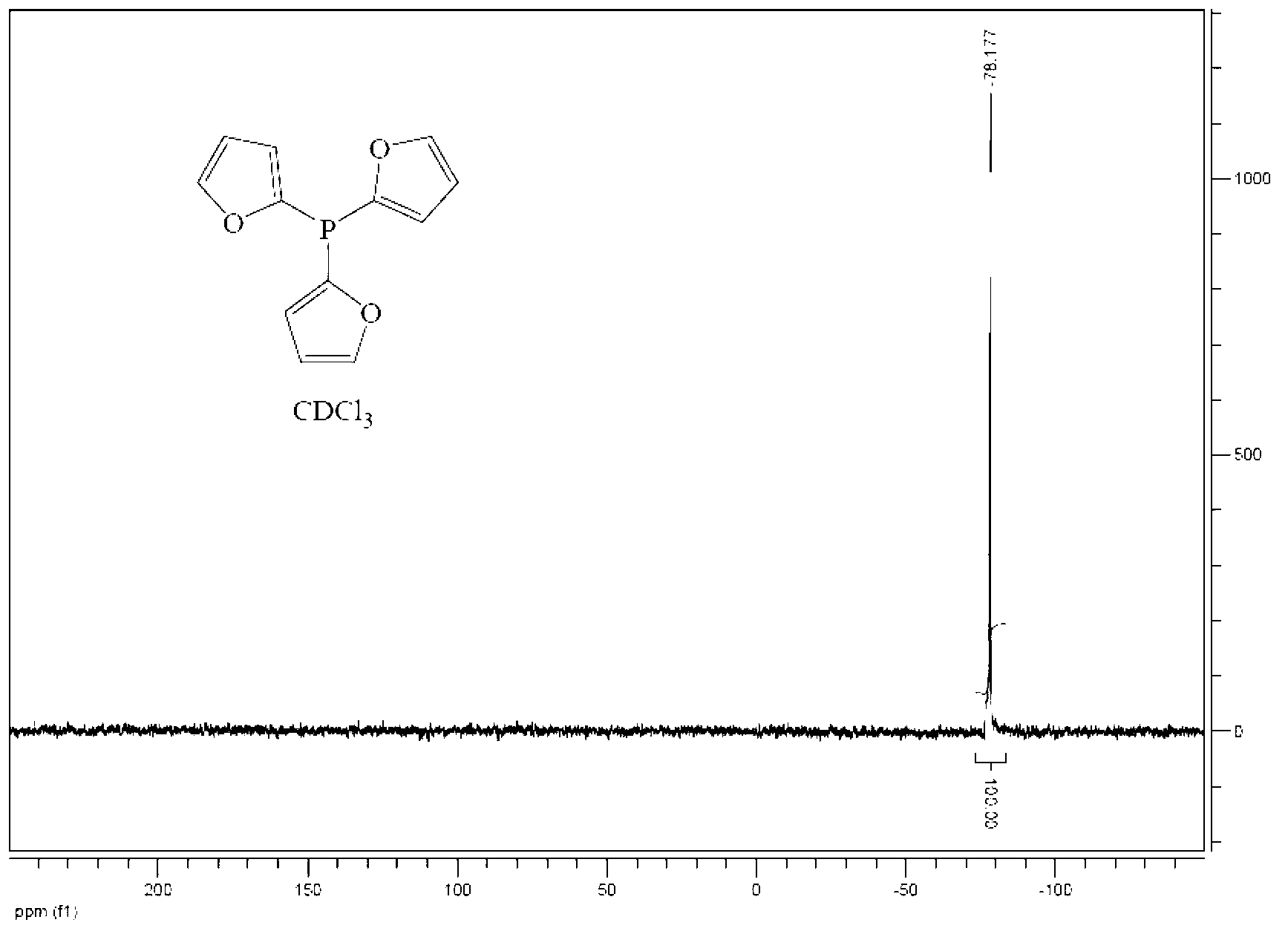
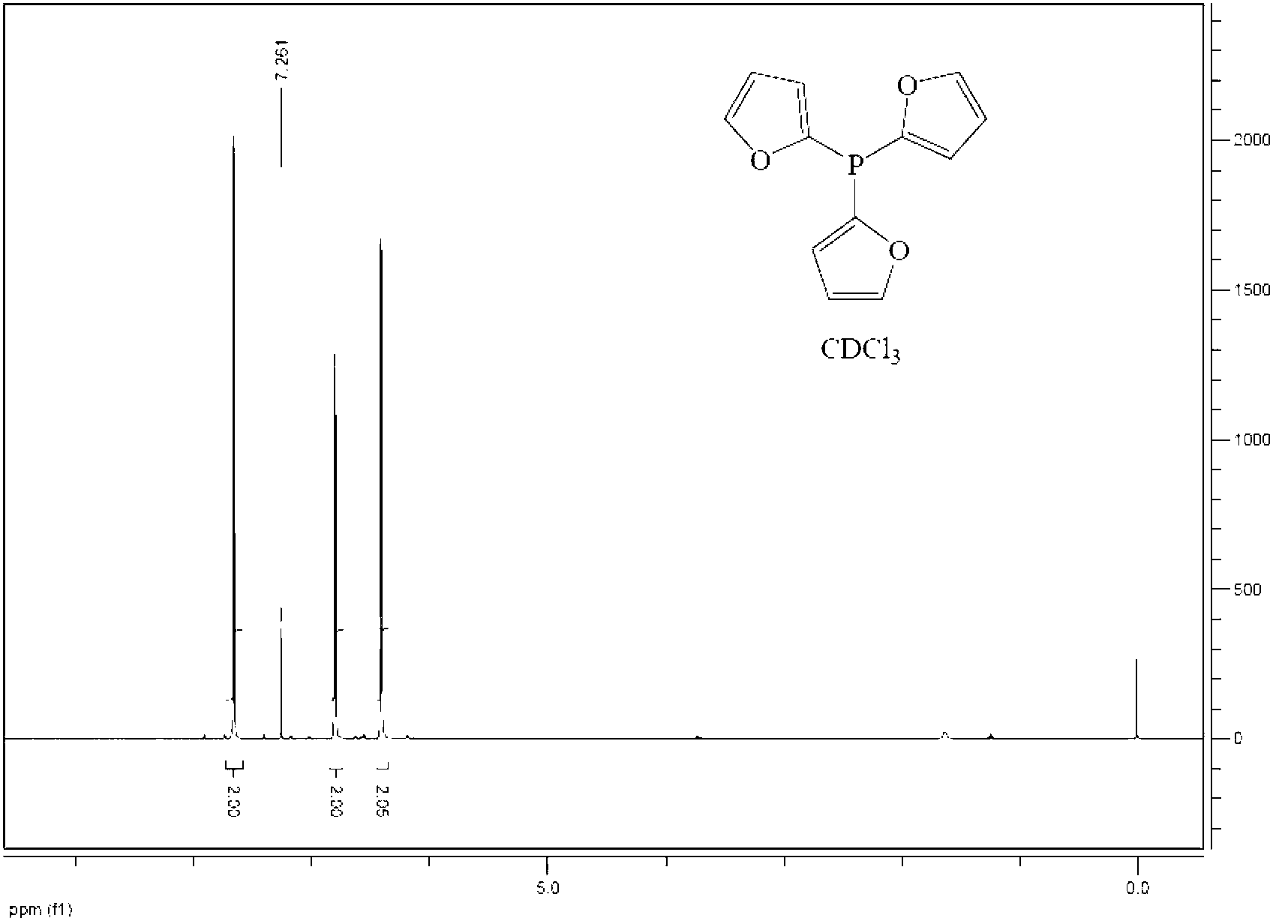
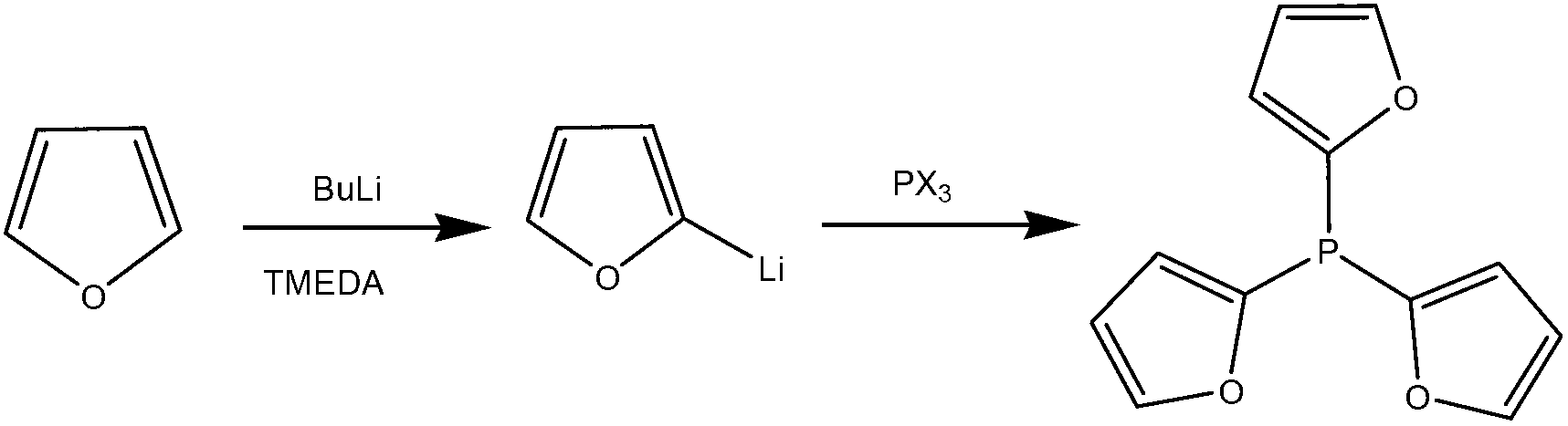
Preparation method of tris(2-furyl) phosphine
A technology of furyl and furan, which is applied in the field of preparation of triphosphorus, can solve the problems of low yield and high cost of raw materials, and achieve the effects of reducing production cost, enhancing lithiation ability, and complete lithiation reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] (1) Preparation of lithium furyl
[0034] Replace the reaction bottle with an inert gas such as a nitrogen atmosphere, and add furan, tetramethylethylenediamine and n-hexane to the reaction bottle under mechanical stirring. The amount of solvent n-hexane is usually added according to the ratio of 500 mL of n-hexane required for 1 mol of furan; At room temperature, add dropwise the butyllithium with a concentration of 1.3-2.5M / L, wherein the mol ratio of butyllithium to furan can be 1:0.9-1, and the mol ratio of butyllithium to tetramethylethylenediamine can be 1:1-2, after the dropwise addition, raise the temperature to 40-60°C, preferably to 45-55°C, keep it for 0.5-3 hours, preferably keep it warm for 1-2 hours.
[0035] (2) Preparation of tri(2-furyl)phosphorus
[0036] Add the n-hexane solution of phosphorus trihalide dropwise to the generated lithium furyl at -10-0°C. The amount of n-hexane as a solvent is usually added in the proportion that 1 mol of phosphorus t...
Embodiment 1
[0048] The reaction flask was replaced with a nitrogen atmosphere, and under mechanical stirring, 20 g of analytically pure furan, 34.8 g of analytically pure TMEDA and 140 mL of n-hexane were added to the reaction flask, and at room temperature, a concentration of 2.5 M / L n-butyl lithium ( Analytical pure) 120mL, after the dropwise addition, the temperature was raised to 50°C and kept for 2h.
[0049] Add analytically pure PCl dropwise at 0°C 3 8g of n-hexane 50mL solution, after the dropwise addition is completed, keep at this temperature for 1h, naturally rise to room temperature, and stir for 5h.
[0050] Pour the reaction solution into 200mL of saturated ammonium nitrate aqueous solution, after fully stirring, let it stand for stratification, separate the organic phase, extract the aqueous phase with dichloromethane for 2-3 times, combine the organic phase, and concentrate the organic phase to obtain the crude product. The product was recrystallized with petroleum ether ...
Embodiment 2
[0055] Replace the reaction bottle into a nitrogen atmosphere, add 20g of analytically pure furan, 27.9g of analytically pure TMEDA and 140mL of n-hexane to the reaction bottle under mechanical stirring, and add 120mL of n-butyllithium with a concentration of 2.5M / L dropwise at room temperature , after the dropwise addition was completed, the temperature was raised to 50°C for 2h.
[0056] Add analytically pure PCl dropwise at 0°C 3 8g of n-hexane 50mL solution, after the dropwise addition is completed, keep at this temperature for 1h, naturally rise to room temperature, and stir for 5h.
[0057] Pour the reaction solution into 200mL of saturated ammonium nitrate aqueous solution, after fully stirring, let it stand for stratification, separate the organic phase, extract the aqueous phase with dichloromethane for 2-3 times, combine the organic phase, and concentrate the organic phase to obtain the crude product. The product was recrystallized with petroleum ether to obtain 10....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com