Method for detecting monosaccharide component in rainbow conk glycopeptide
A technology of versicolor glycopeptide and detection method, which is applied in the directions of measuring devices, material separation, and analysis materials, etc., which can solve problems such as failure to achieve effective separation, and achieve the effect of ensuring quality and ensuring curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
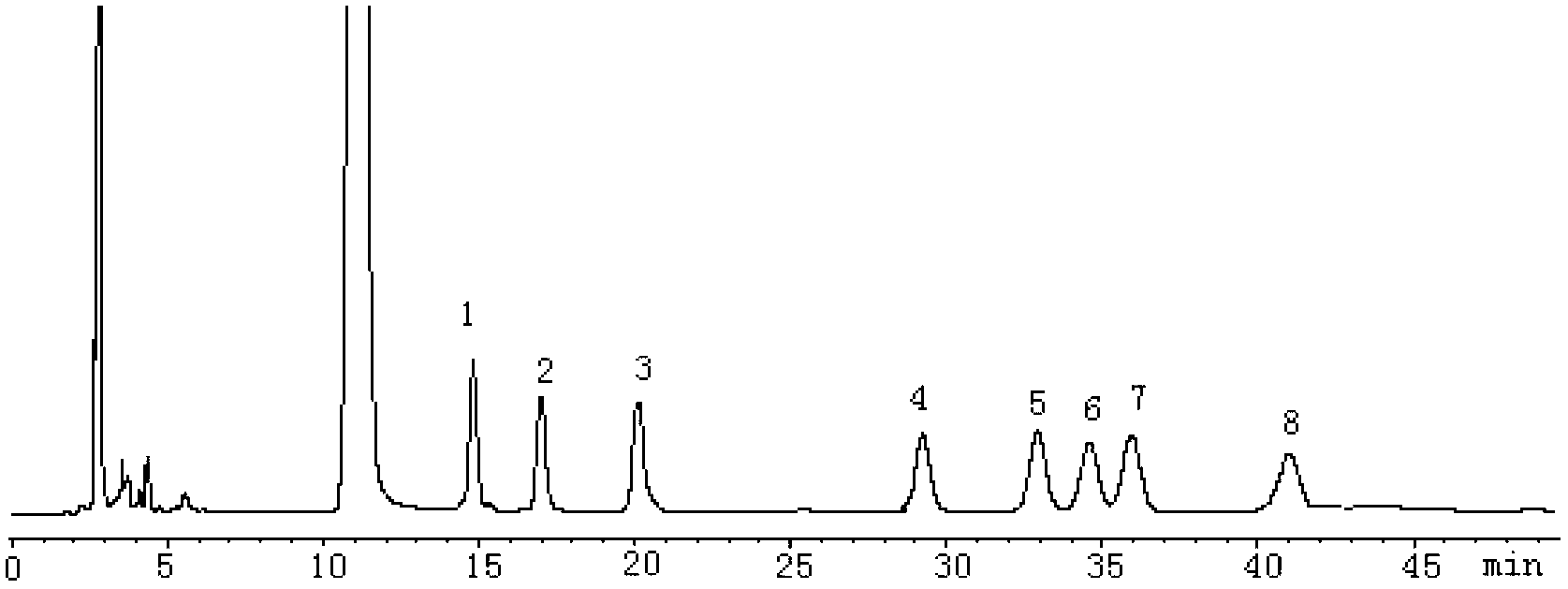
[0024] According to Chinese Pharmacopoeia 2010 Edition (Part One) Appendix VI D high performance liquid chromatography.
[0025] Chromatographic conditions and system suitability test: use Phenomenex Luna C 18 (4.6mm×250mm, 5μm) column; the mobile phase is acetonitrile-phosphate buffer solution, the volume percentage of acetonitrile is 18%, the concentration of phosphate buffer solution is 0.05mol / L, the pH value of phosphate buffer solution is 7.0, UV detection The detector detection wavelength is 255nm, and the number of theoretical plates is 6560 based on the glucose 1-phenyl-3-methyl-5-pyrazolone derivative.
[0026] Preparation of mixed reference solution: Accurately weigh D-glucose, D-mannose, D-xylose, L-fucose, D-galactose, D-arabinose, D-hydrochloric acid dried at 105°C to constant weight Add appropriate amount of glucosamine and L-rhamnose reference substances, add water to prepare a mixed reference solution with a concentration of each monosaccharide of 2mmol / L, ta...
Embodiment 2
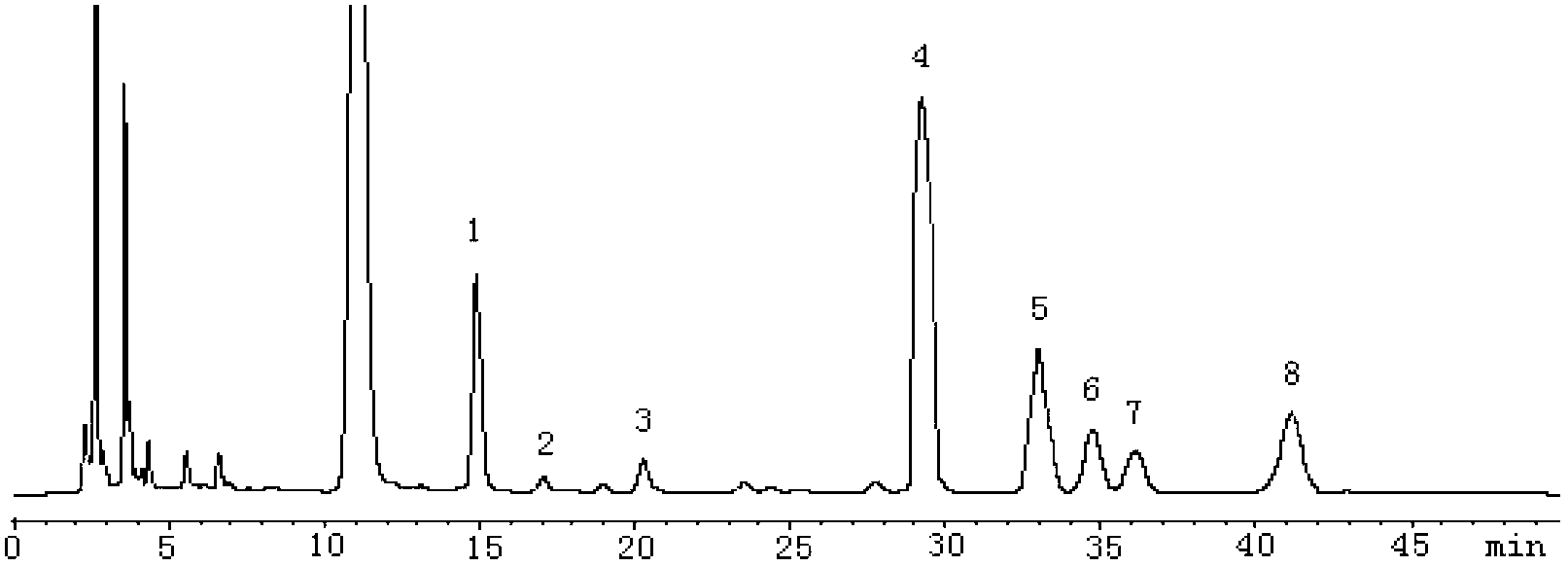
[0030] According to Chinese Pharmacopoeia 2010 Edition (Part One) Appendix VI D high performance liquid chromatography.
[0031] Chromatographic conditions and system suitability test: use Hypersil BDS C 18 (4.6mm × 200mm, 5 μm) column; mobile phase is acetonitrile-phosphate buffer, wherein the volume percentage of acetonitrile is 16%, the concentration of phosphate buffer is 0.03mol / L, the pH value of phosphate buffer is 6.9, ultraviolet detection The detector detection wavelength is 250nm, and the number of theoretical plates is 8308 based on the glucose 1-phenyl-3-methyl-5-pyrazolone derivative.
[0032] Preparation of mixed reference substance solution: the same as the preparation method of mixed reference substance standard solution in Example 1.
[0033] Preparation of the test solution: take 10 Yunzhi glycopeptide capsules, pour out the contents and grind them finely in a mortar, accurately weigh 5.0 mg and put them in a 5 mL ampoule, add 1 mL 4mol / L trifluoroacetic ac...
Embodiment 3
[0036] According to Chinese Pharmacopoeia 2010 Edition (Part One) Appendix VI D high performance liquid chromatography.
[0037] Chromatographic conditions and system suitability test: use Phenomenex Gemini C 18 (4.6mm×250mm, 5μm) column; the mobile phase is acetonitrile-phosphate buffer, the volume percentage of acetonitrile is 20%, the concentration of phosphate buffer is 0.07mol / L, and the pH of phosphate buffer is 7.1 , the detection wavelength of the ultraviolet detector is 260nm, and the number of theoretical plates is calculated as 12310 based on the glucose 1-phenyl-3-methyl-5-pyrazolone derivative.
[0038] Preparation of mixed reference substance solution: the same as the preparation method of mixed reference substance standard solution in Example 1.
[0039] Preparation of the test solution: Take 10 pieces of Yunzhi glycopeptide tablets and grind them finely in a mortar, accurately weigh 5.0mg and place them in a 5mL ampoule, add 1mL 2mol / L trifluoroacetic acid and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com