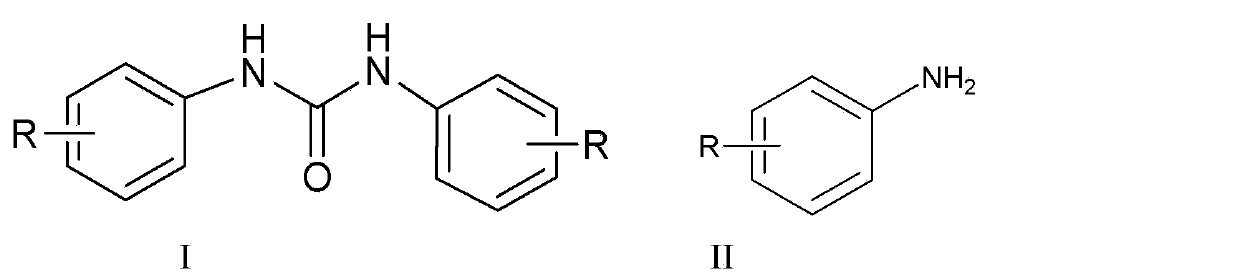
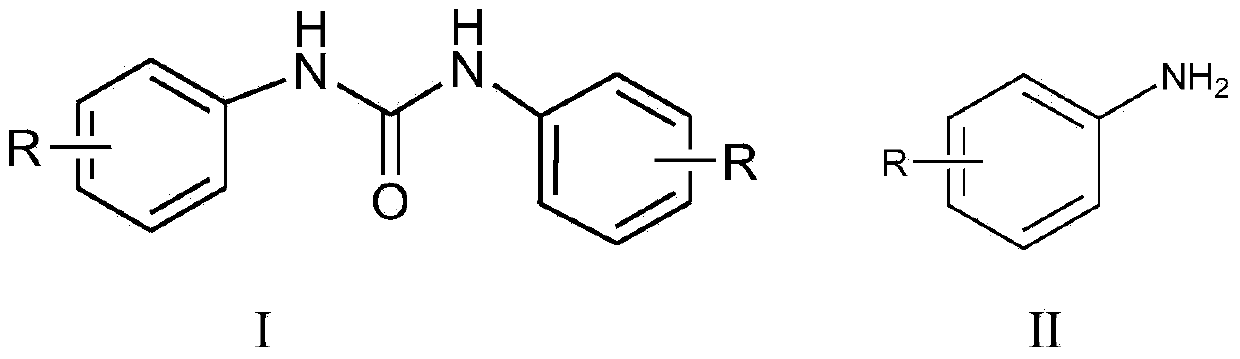
Synthetic method for diaryl urea compound
A technology for diarylurea and aniline compounds, which is applied in the field of synthesis of diarylurea compounds, can solve the problems of difficult purification of products, harsh reaction conditions of diarylureas, etc., and achieves short reaction time, convenient post-processing, simple method effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Take 2.79 g of aniline and 2.97 g of triphosgene in a 250 mL three-necked flask, add 40 mL of dichloromethane, add 3.03 g of triethylamine dropwise in an ice bath, react at room temperature after the dropwise addition, and track and detect with TLC. The reaction is complete in about 1 hour. 35 mL of dichloromethane solvent was recovered by distillation, the residue was washed with 20 mL of water, filtered, and the filter cake was dried to obtain 3.08 g of N,N'-diphenylurea solid product, yield 97%, melting point m.p. 245.0-245.4 °C, Purity 98.4% (HPLC)
Embodiment 2
[0029] Take 2.79 g of aniline and 2.97 g of triphosgene in a 250 mL three-neck flask, add 40 mL of tetrahydrofuran, add 3.50 g of tri-n-propylamine dropwise under ice bath, react at room temperature after the dropwise addition, and track and detect with TLC. After 1 h, the reaction is complete, and the solvent is recovered by distillation Tetrahydrofuran was 37 mL, the residue was washed with 20 mL of water, filtered, and the filter cake was dried to obtain 3.02 g of N,N'-diphenylurea solid product, yield 95%, melting point m.p. 245.0-245.4 °C, purity 97.8% ( HPLC).
Embodiment 3
[0031] Take 3.21 g of 2-methylaniline and 2.97 g of triphosgene in a 250 mL three-necked flask, add 50 mL of ethyl acetate, add 3.03 g of triethylamine dropwise under ice-cooling, react at room temperature after the dropwise addition, and track and detect with TLC for 2 h After the reaction was complete, the ethyl acetate solvent was recovered by distillation. The residue was washed with 20 mL of water, filtered, and the filter cake was dried to obtain 3.52 g of 1,3-di-o-toluene urea as a solid. Ethanol was recovered by distillation with a yield of 98% and a melting point of 258.4- 258.9 °C, 98.5% purity (HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com