Coumarin derivative and preparation method and application in detecting cyanide ion
A technology of coumarins and cyanide ions, which is applied in the direction of chemical reaction analysis of materials, measurement of color/spectral characteristics, material analysis by observing the influence of chemical indicators, etc., to achieve good selectivity and expand High detection range and high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Preparation and Application of Coumarin Derivatives (A)
[0031]
[0032] (1) In a dry 50 mL round bottom flask, add 0.91 g (6.6 mmol) 2,4-dihydroxybenzaldehyde, 0.6 mL (4 mmol) diethyl malonate, 10 mL absolute ethanol, 0.1 mL Hexahydropyridine and 2 drops of glacial acetic acid were heated and stirred at 80°C under reflux for 6 h, crystals precipitated after cooling, the solid was collected by suction filtration, washed with absolute ethanol, and dried to obtain the product ethyl 7-hydroxycoumarin-3-carboxylate.
[0033] (2) In a dry 25 mL round bottom flask, add 1.64 g (7.0 mmol) ethyl 7-hydroxycoumarin-3-carboxylate, 1.5 g (10.7 mmol) urotropine (hexamethylene tetra amine) and 10 mL of trifluoroacetic acid, heated and stirred at reflux at 85°C for 24 h, after cooling, add 10 mL of 20% (volume fraction) H 2 SO 4 Stir for 1h. Pour the reaction solution into a 50 mL beaker, add 25 mL of water, let it stand still, crystals precipitate out, collect the sol...
Embodiment 2
[0035] Example 2 Preparation of Coumarin Derivatives (A)
[0036] (1) In a dry 50 mL round bottom flask, add 0.91 g (6.6 mmol) 2,4-dihydroxybenzaldehyde, 0.6 mL (4 mmol) diethyl malonate, 10 mL absolute ethanol, 0.1 mL Hexahydropyridine and 2 drops of glacial acetic acid, heated and stirred at 85°C under reflux for 6 h, crystals precipitated after cooling, the solid was collected by suction filtration, washed with absolute ethanol, and dried to obtain the product ethyl 7-hydroxycoumarin-3-carboxylate.
[0037] (2) In a dry 25 mL round bottom flask, add 1.64 g (7.0 mmol) ethyl 7-hydroxycoumarin-3-carboxylate, 1.5 g (10.7 mmol) urotropine (hexamethylene tetra amine) and 10 mL of trifluoroacetic acid, heated and stirred at reflux at 85°C for 24 h, after cooling, add 10 mL of 20% (volume fraction) H 2 SO 4 Stir for 1h. Pour the reaction solution into a 50 mL beaker, add 25 mL of water, let it stand still, crystals precipitate out, collect the solid by suction filtration, and dr...
Embodiment 3
[0039] Example 3 Application of Coumarin Derivatives (A)
[0040] Determination data of coumarin derivatives (A) prepared by Examples (1) and (2):
[0041] 1 H NMR (400 MHz, DMSO-d 6 ): δ 1.31 (t, J = 6.8 Hz, 3H), 1.79 (s, 6H), 2.33 (s, 1H), 2.67 (s, 1H), 4.01 (s, 3H), 4.29 (q, J = 6.0 Hz, 2H), 7.62 (s, 2H), 7.89 (s, 2H), 8.13 (d, J = 16.0 Hz, 1H), 8.52 (d, J = 16.0 Hz, 1H), 8.72 (s, 1H). FT-IR (KBr, cm -1 ): 3444, 2989, 2451, 1771, 1706, 1601, 1539, 1476, 1373, 1284, 1250, 1026, 832, 751, 681. HRMS (ESI): m / z, calcd for (M + ) 418.1600; Found 418.1598.
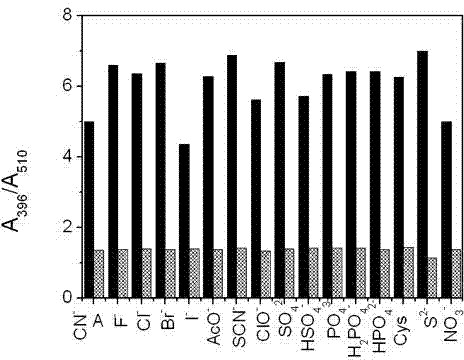
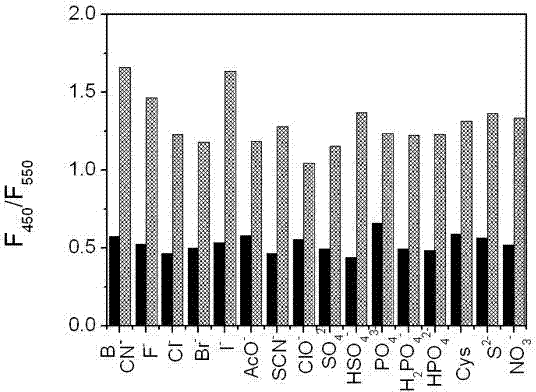
[0042] UV spectrum test of coumarin derivatives (A) on cyanide ion concentration: add 0.5 mL of 100 μM solution of coumarin derivatives (A) to a 10 mL colorimetric tube, 1 mL of anhydrous methanol, 1 mL buffer solution (Na 2 CO 3 -NaHCO 3, pH = 9.4), and then add concentrations of 0, 10, 20, 40, 60, 80, 95, 150, 200, 300, 400, 500, 600, 700, 900, 1000, 2000, 4000, 6000, 10000 μM sodium cyanide solution. After cons...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com