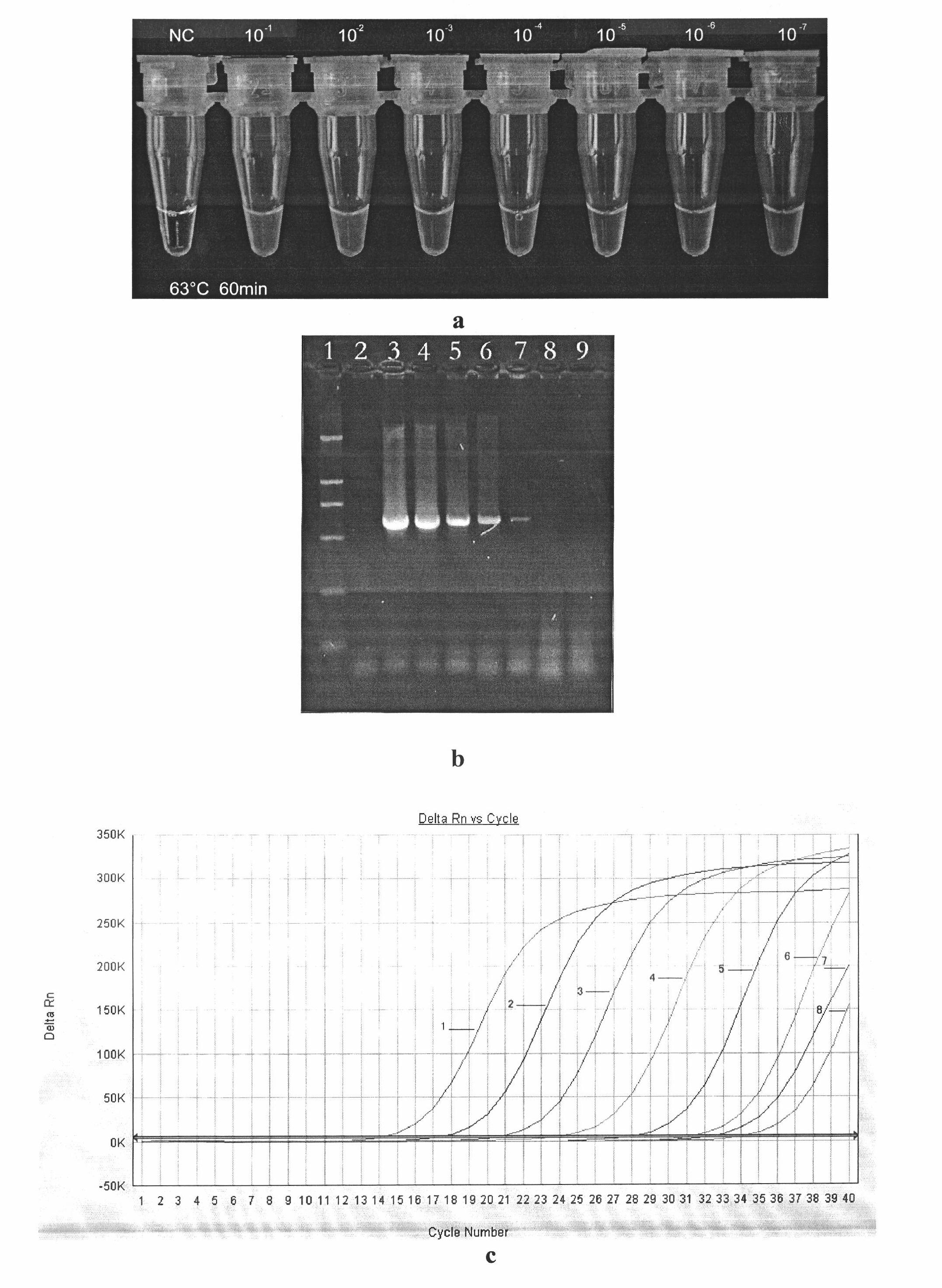
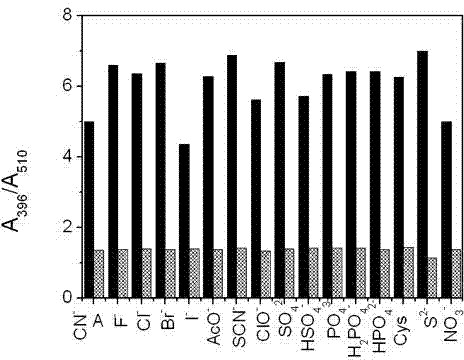
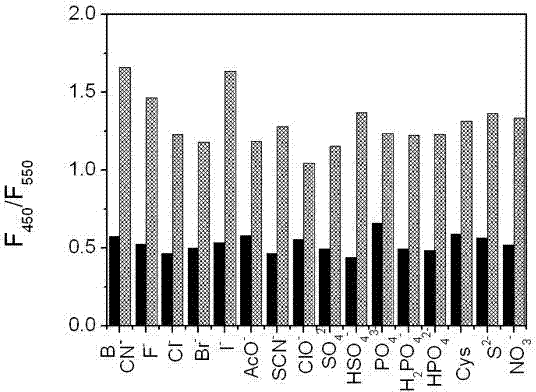
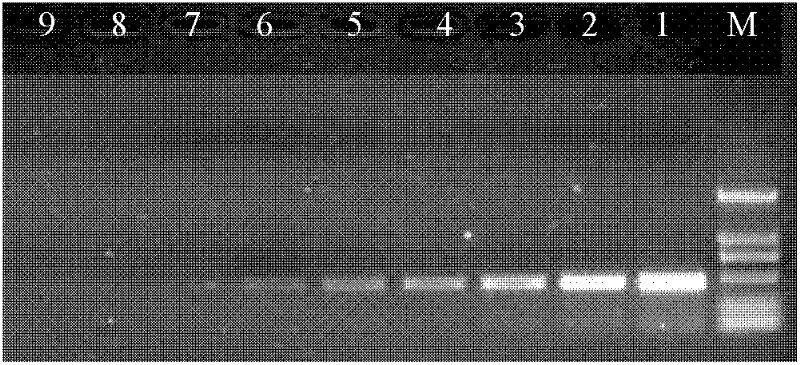
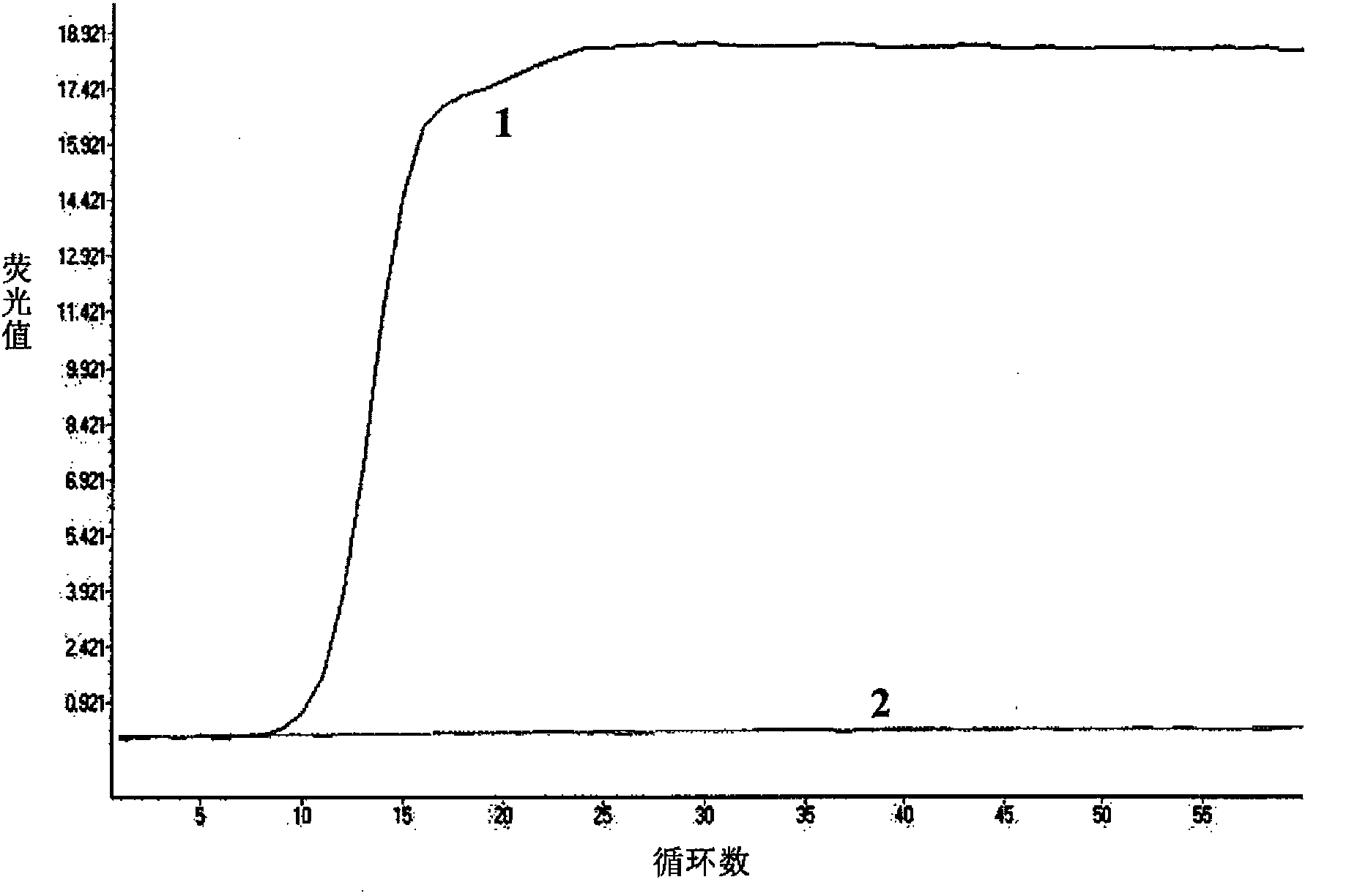
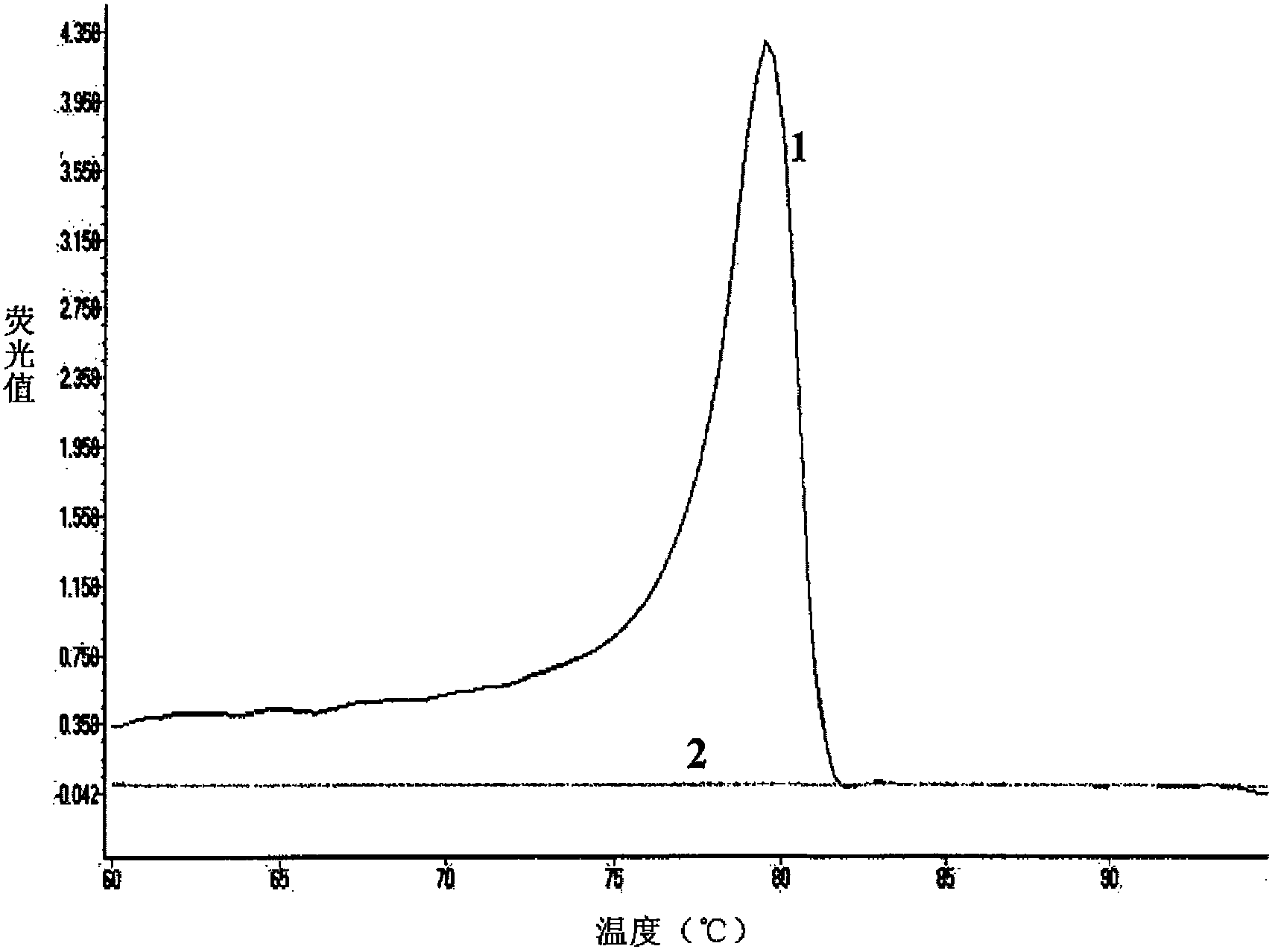
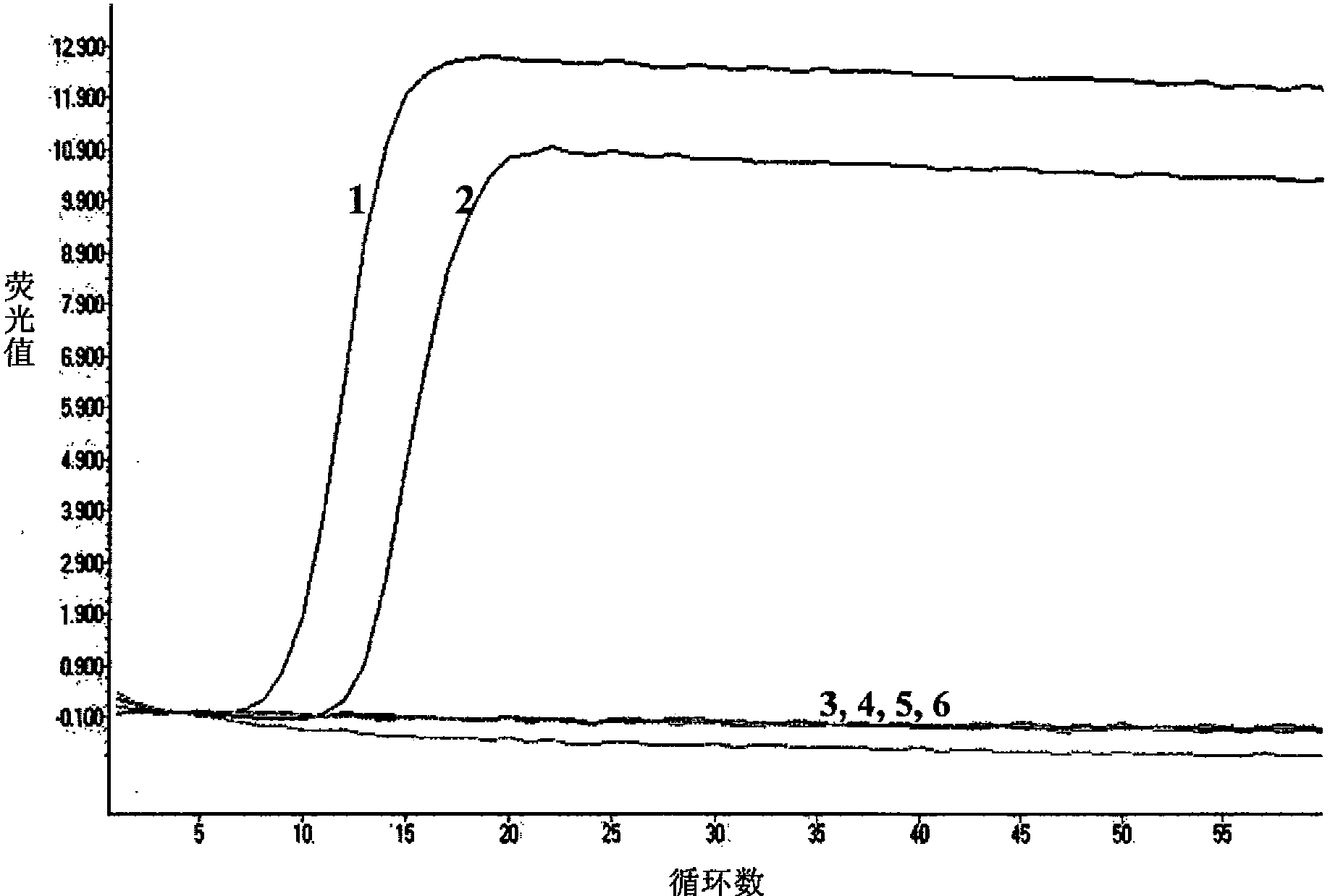
Patents
Literature
35results about How to "Specific detection method" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Primer for amplifying novel coronavirus and application of primer
InactiveCN111270016ASensitiveStrong conservativeMicrobiological testing/measurementAgainst vector-borne diseasesPrimary screeningOligonucleotide Primer
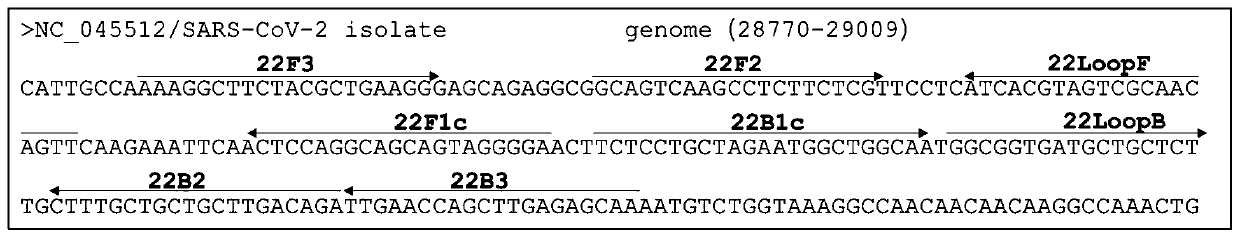
The invention relates to a primer for amplifying novel coronavirus and an application thereof. The primer comprises at least one oligonucleotide primer capable of recognizing a specific region on a novel coronavirus N gene, and the sequence range of the specific region on the novel coronavirus N gene comprises a region sequence of a novel coronavirus reference strain genome 28778-28971. By adopting the primer, novel coronavirus nucleic acid can be rapidly detected from RNA extracted from samples such as blood, saliva, sputum and air; the method has the advantages of simplicity, convenience, quickness and no need of training of large instruments and professionals, can be used as a primary screening and environment detection method for novel coronavirus infected suspected pneumonia cases, and can also be used as an important reference for clinical diagnosis.
Owner:LUDONG UNIVERSITY
A detecting method and medium of bacillus coagulans
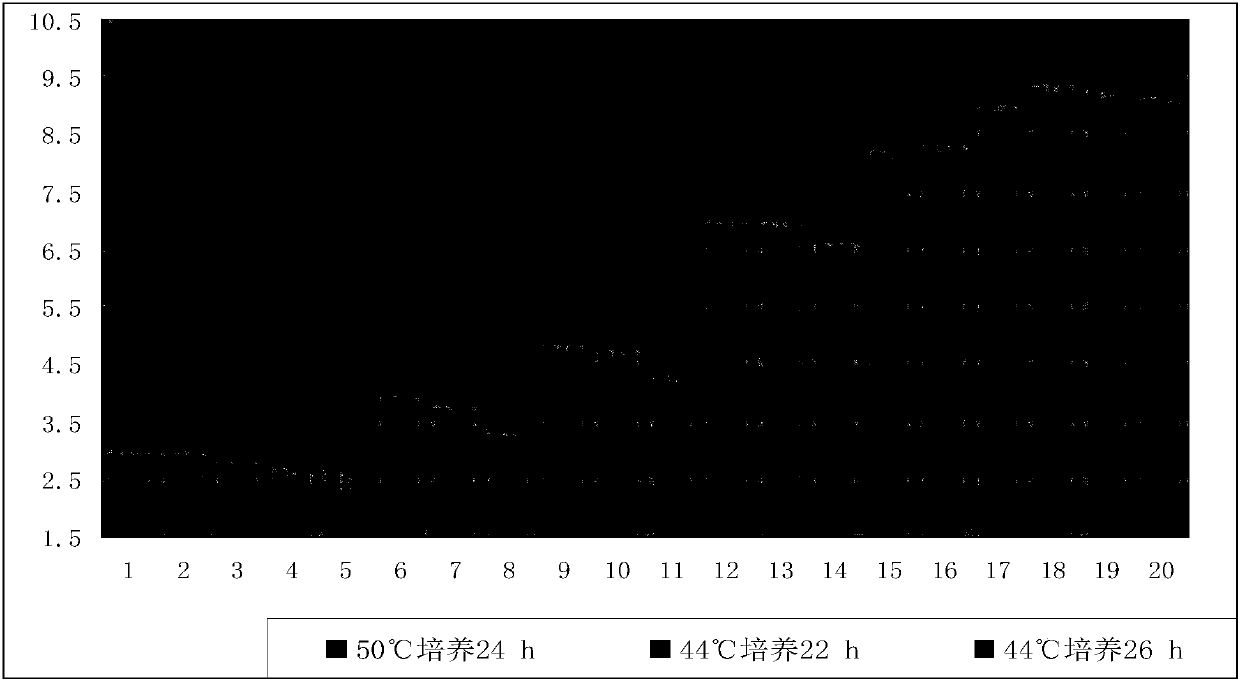
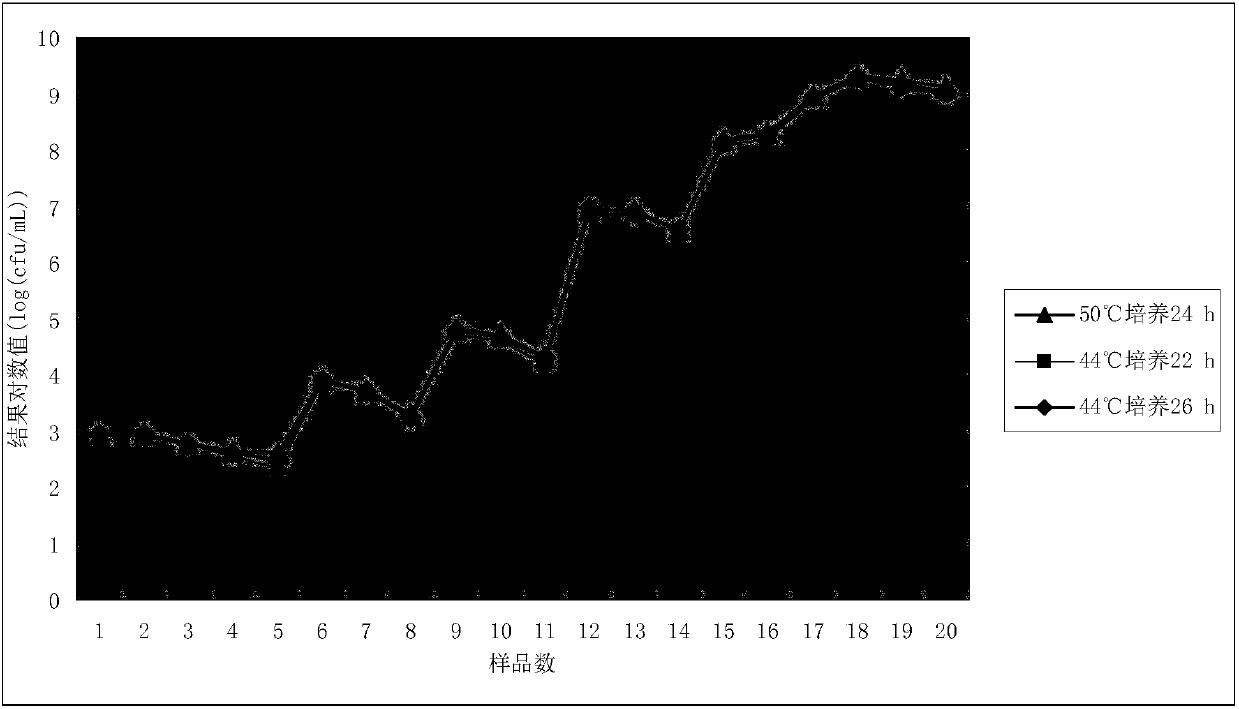
ActiveCN108034607ARich detection methodsSpecific detection methodBacteriaMicrobiological testing/measurementD-GlucoseGlucose polymers
The invention relates to a detecting method and medium of bacillus coagulans. The medium includes 5.0-10.0 parts by weight of tryptone, 2.0-3.0 parts by weight of yeast extract powder / extract, 1.0-5.0parts by weight of glucose, 1.0-2.0 parts by weight of polysorbate-80, 0.1-0.5 part by weight of L-cysteine, 0.04-0.06 part by weight of bromocresol purple, and 15.0-20.0 parts by weight of agar. Themedium allows colonies to grow well, and is low in cost and simple and convenient to prepare. A counting result is accurate and reliable when the medium is utilized for detection of the bacillus coagulans.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Loop-mediated isothermal amplification (LAMP) detection method and kit of soft-shelled turtle iridovirus (STIV)
InactiveCN102146483AFast detection methodSensitive detection methodMicrobiological testing/measurementSpecific detectionMicrobiology
The invention discloses a loop-mediated isothermal amplification (LAMP) detection method and a kit of soft-shelled turtle iridovirus (STIV). An outer primer comprises sequences shown as Seq ID No.1 and Seq ID No.2. A quick, sensitive and specific detection method for detecting the STIV is provided, field instant detection of viruses can be performed, and technical support is provided for healthy cultivation of soft-shelled turtles and the development of international trade.
Owner:SHENZHEN AUDAQUE DATA TECH
Coumarin derivative and preparation method and application in detecting cyanide ion
ActiveCN103044406AEnhanced nucleophilicityObvious color changeOrganic chemistryMaterial analysis by observing effect on chemical indicatorWastewaterStructural formula
The invention discloses a ratio-type coumarin derivative which can be used for detecting cyanide ions. The structural formula (I) of the coumarin derivative is described in the specification, wherein R is shown in the specification. The coumarin derivative is used for detecting cyanide ions; the process of detecting the cyanide ion is carried out in a buffer solution of Na2CO3-NaHCO3; and the pH value of the buffer solution is 9.4. The coumarin derivative provided by the invention can be used for uniquely, efficiently and simply detecting the cyanide ions, is applicable to detection on cyanide ions in wastewater generated in industrial processes such as electroplating, chemical engineering, metallurgy, mineral separation and surface treatment, and is high in detection sensitivity and good in selectivity.
Owner:山西永津集团有限公司
Para-gene sequence for exogenous insertion vector of corn strain MON863
InactiveCN1740322ASpecific detection methodMicrobiological testing/measurementFermentationNADH dehydrogenaseTobacco mosaic virus TMV
This invention relates to the side gene sequence of MON863 maize exogenous inserted carrier. It includes: partial nucleotide sequences which codes maize NADH dehydrogenase subunit 1and 2. This nucleotide sequence is totally identical with either 1-253 nucleotide sequence in the SEQ ID NO.1 or 138-411 nucleotide sequence in the SEQ ID NO.2. The partial sequences of 35S promoter, which code the tobacco mosaic virus, come from exogenous inserted carrier PV-ZMIR13 and have the same nucleotide sequence with 254-456 nucleotide sequence in the SEQ ID NO.1. The partial sequences, which code the wheat tahsp 17 3í» terminator, come from exogenous inserted carrier PV-ZMIR13 and have the same nucleotide sequence with 1-137 nucleotide sequence in the SEQ ID NO.2.
Owner:SHANGHAI JIAO TONG UNIV
RT-HDA kit and primer for detecting avian influenza virus
ActiveCN102912038AImprove securityRapid responseMicrobiological testing/measurementDNA/RNA fragmentationOligonucleotidePolymerase chain reaction
The invention discloses an RT-HDA kit for detecting avian influenza virus and a primer. The primer for detecting the avian influenza virus is oligonucleotides sequences shown in a sequence table SEQ ID (Sequence ID) NO: 1 and a sequence table SEQ ID NO:2. According to the RT-HDA kit, a novel constant-temperature amplifying technology HDA is used for detecting the avian influenza virus for the first time; compared with a conventional detection method of AIV (Avian Influenza Virus), the RT-HAD kit has the advantages that: (1) an HDA detection method is faster, more convenient, safer and more reliable. A conventional method of separating virus and a conventional serology method need more time, and bring a risk to safety of an organism; the HDA method simulates a natural replication mechanism in vivo, so that the safety is high, a reaction is fast to carry out, and detection can be accomplished by only two hours; and (2) compared with a PCR (Polymerase Chain Reaction), the HDA detection method has the advantages that PCR instruments are saved, a requirement on equipment is simple, only one water bath kettle is needed, so that the technology is convenient to master and operate, the detection method can be convenient to popularize and use in regions with insufficient conditions, and benefits are provided for diagnosing and monitoring the avian influenza virus.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Pythium splendens braun fluorescence quantitative PCR detection reagent and detection kit and application
InactiveCN102382890AAccurate qualitative and quantitative detectionEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceNegative controlElectrophoresis
The invention discloses a pythium splendens braun fluorescence quantitative PCR detection reagent, a detection kit and an application; the detection reagent comprises a pair of specific primers with sequences as shown in SEQ ID NO: 1 and SEQ ID NO: 2, and a specific fluorescence probe with a sequence as shown in SEQ ID NO: 3; an amplification target fragment has a length of 94 bp, and the nucleotide sequence of the amplification target fragment is shown in SEQ ID NO: 4; the detection kit comprises components of a CTAB extract, a PCR buffer containing the primers and the probe, TaqDNA polymerase, positive control liquid, and quantitative standard liquid; the detection method comprises the extraction of total RNA and the fluorescence PCR reaction; detection performed by using the pythium splendens braun fluorescence quantitative PCR detection reagent overcomes disadvantages of time consumption, easy pollution, and electrophoresis detection necessity after amplification of routine PCR, can realize rapid and accurate qualitative and quantitative detection of pythium splendens braun in a sample, and has the advantages of simplicity, easy operations, intuitive results, high sensitivity, good repeatability, and the like.
Owner:NINGBO ACAD OF SCI & TECH FOR INSPECTION & QUARANTINE
Eu-labelling early diagnosis kit for cytomegalovirus infection
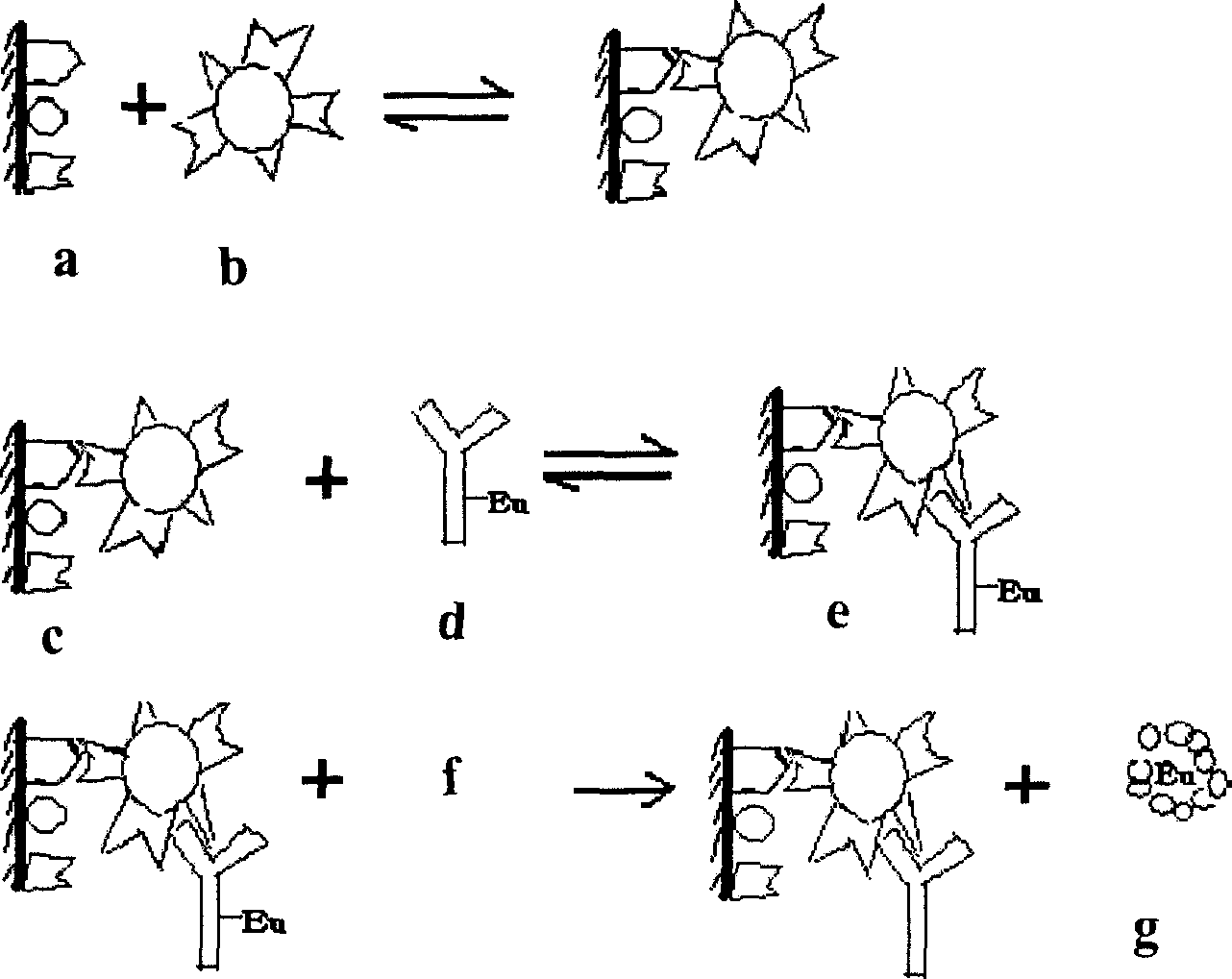
InactiveCN1731187AStrong specificityEliminate distractionsMaterial analysisOperating instructionCytomegalovirus infections
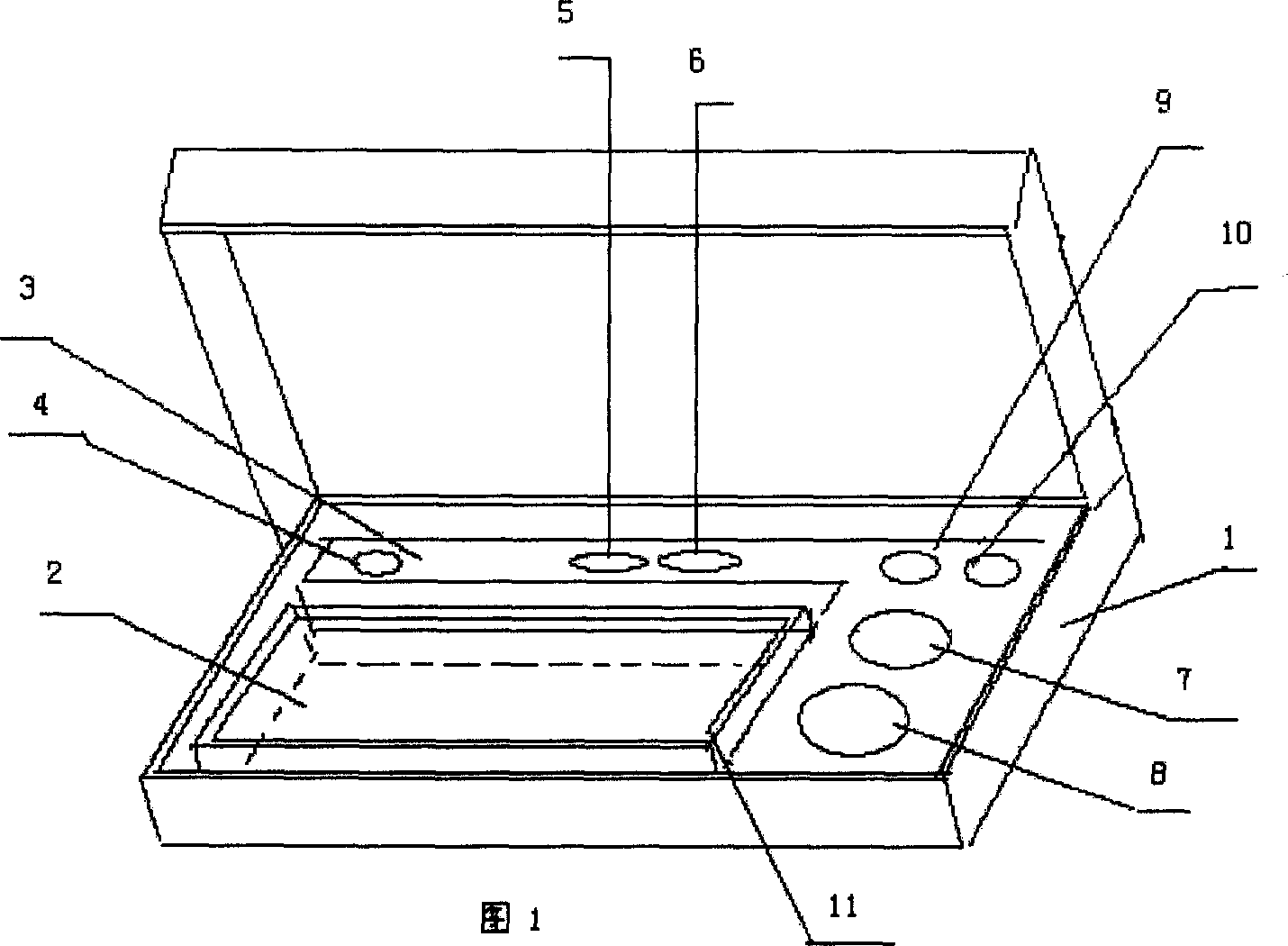
The invention provides an enropin label idioldast viral infection early stage quotation which is formed by a box 1, a uterus plate 2, a pad 3, an enropin label antibody 4, a negative nature control object 6, a dilution buffer liquid 7, a reacting buffer liquid 8, a washing buffer liquid 9, a reinforcing liquid 10, and an operating instruction 11.
Owner:ZHEJIANG UNIV
LAMP primer combination, detection method and kit capable of distinguishing porcine circovirus type 2 and type 3 typing detection
InactiveCN110423846AAvoid pollutionSpecific detection methodMicrobiological testing/measurementDNA/RNA fragmentationHighly pathogenicCircovirus
The present invention belongs to the technical field of biology and discloses a LAMP primer combination, detection method and kit capable of distinguishing porcine circovirus type 2 and type 3 typingdetection. The primer combination can respectively detect the porcine circovirus type 2 and the porcine circovirus type 3, and also has no cross reactions with other porcine viruses (such as circovirus type 1, porcine parvovirus, pseudorabies virus, porcine cholera virus, swine influenza virus, highly pathogenic porcine reproductive and respiratory syndrome virus, etc.). Fluorescent dye is added into a LAMP reaction system, so that detection of the porcine circovirus type 2 and / or type 3 can be realized by a one-step method, whole-process real-time monitoring is realized by combining fluorescence quantification, results can be obtained within 1 hour, and detection sensitivity can reach 100 copies / [mu]L. The detection method has advantages of simplicity, convenience, rapidness, sensitivityand specificity, and also avoids errors caused by artificial judgment and environmental pollution caused by uncovering.
Owner:CAPITALBIO CORP +1
Application of monoclonal antibody for Riemerella anatipestifer in preparation of immune colloidal gold test paper
InactiveCN102435742AFast detection methodSpecific detection methodImmunoglobulins against bacteriaMicroorganism based processesDiseaseBiochemistry
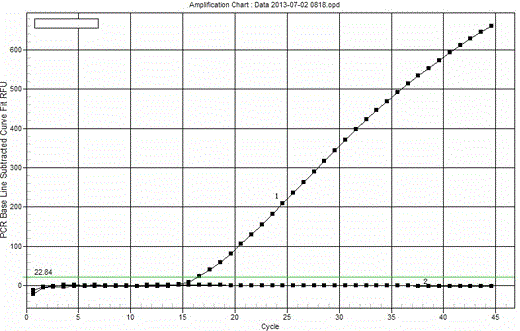
The invention belongs to the field of biotechnology and particularly relates to a monoclonal antibody for Riemerella anatipestifer and an application thereof. The monoclonal antibody 5G7 has a preservation number of CGMCC (China General Microbiological Culture Collection Center) No.5165. The invention also discloses an application of the monoclonal antibody in preparation of an immune colloidal gold test paper for detecting the Riemerella anatipestifer. According to the invention, the immune colloidal gold test paper can be used to not only detect cause of disease directly but also detect RA (Riemerella Anatipestifer) strains of different serotypes 1, 2, 3 and 11 at the same time, so that the immune colloidal gold test paper has higher application value.
Owner:YANGZHOU UNIV
Drug resistance detection method for vibrio parahaemolyticus fluoroquinolone medicines
InactiveCN111206068ANo time consuming trainingSensitive detection methodMicrobiological testing/measurementMicroorganism based processesVibrio parahaemolyticusGel electrophoresis
The invention relates to a drug resistance detection method for vibrio parahaemolyticus fluoroquinolone medicines. The method comprises the following steps: (1) collecting 1.0-1.2g of live bacteria ofvibrio parahaemolyticus, performing resuspension by using 7-8mL of deionized water, performing repeated freezing and thawing to break cells, performing centrifugation, and collecting supernate so asto obtain a template; (2) commixing the template with fluoroquinolone medicine resistant gene detection primers GyrA-F, GyrA-R, GyrB-F, GyrB-R, ParE-F and ParE-R, and performing PCR (polymerase chainreaction) amplification; and (3) after PCR amplification is completed in the step (2), performing agarose gel electrophoresis and sequencing. The detection method provided by the invention is sensitive, specific and rapid, a very trace amount of target genes can be detected, and tedious vibrio parahaemolyticus culture is not needed.
Owner:HUBEI UNIV OF ARTS & SCI
Fluorescence RT-PCR primer, probe and kit for detecting Schmallenberg viruses
InactiveCN104450959ASpecific detection methodQuick checkMicrobiological testing/measurementMicroorganism based processesRNA extractionReverse transcriptase
The invention discloses a fluorescence RT-PCR primer, a probe and a kit for detecting Schmallenberg viruses. The sequences of the primer and the probe are shown as SEQ ID NO. 1-6. The kit comprises the primer, the probe, reverse transcriptase, DNA polymerase, an RNA extraction reagent, a fluorescence PCR reaction reagent, a Schmallenberg virus positive control and a Schmallenberg virus negative control. The Schmallenberg virus fluorescence RT-PCR kit and a detection method provided by the invention are specific, sensitive and quick, have good repeatability and low costs, and the detection method is a favorable method for quickly detecting Schmallenberg viruses.
Owner:中华人民共和国珠海出入境检验检疫局
Reagent and method for detecting iodine through antimony-cerium reaction iodine catalysis method
PendingCN111537462AEasy to useImprove stabilityPreparing sample for investigationColor/spectral properties measurementsNutritionCerium
A classic trace iodine detection method is an arsenic-cerium catalytic spectrophotometric method, such as a sanitary industry standard method WS / T 107-2016 for urine iodine detection, and is widely applied to iodine deficiency and iodine nutrition monitoring in China. However, a highly toxic arsenic trioxide (arsenic trioxide) reagent needs to be used in the method, and the application of the method is increasingly limited due to environmental protection. A non-toxic trace iodine detection reagent and a non-toxic trace iodine detection method are urgently needed in the field of health care. The invention discloses a reagent and a method for detecting iodide ions by adopting an iodine catalyzed antimony-cerium redox reaction principle. Iodine ions catalyze Sb < 3 + > to reduce yellow Ce < 4+ > into colorless Ce < 3 + >, and the reaction speed and the iodine content are in a positive correlation quantitative relation. And after a certain reaction time, the absorbance of the residual Ce< 4 + > in the solution is measured by using a spectrophotometric method, and the iodine content of the measured sample is calculated according to the quantitative relationship between the iodine content and the absorbance. According to the method, toxic-free antimony is adopted to replace highly-toxic arsenic in a classic method, the detection effect consistent with that of an arsenic-cerium catalytic spectrophotometry is achieved, the highly-toxic arsenic trioxide reagent is prevented from being used, and the method is applied to detection of urine iodine, blood iodine, water iodine, salt iodine, food iodine, soil iodine and the like and has important public health significance.
Owner:刘列钧
Method and reagent box for inspecting mycelian protein antibody of white candida
InactiveCN1294418CSpecific detection methodEfficient detection methodImmunoglobulinsMaterial analysisAntigenPeptide
The invention discloses a method and a reagent box for inspecting a candida albicans hypase protein antibody and diagnosing attack infection of candida albicans. The method performs filtering by using a monoclonal antibody of anti-candida albicans hypase protein P47 in a bacteriophage surface display peptide library, prepares simulated antigen or candida albicans hypase protein P47 natural antigen by expanding and purifying, then uses the antigen peridiuming a solid support, adds a biologic sample to be inspected to incubate and wash, adds a marked second antibody for human antibodies to incubate and wash, detects existing of an immune combined composition in an incubated mixture. The reagent box includes an ELISA reagent box and a colloid gold reagent box. The candida albicans hypase protein antibody can be inspected by usign the method simply and in high speed and efficiency; the reagent box has a low cost, convenient usage and high specificity.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A
Real-time fluorescence RT-HDA (Reverse Transcriptase-Helicase-Dependent Isothermal Amplification) kit and primer for detecting avian influenza virus
ActiveCN102876813BFast detection methodThe detection method is convenient and fastMicrobiological testing/measurementMicroorganism based processesReverse transcriptaseFluorescence
The invention discloses a real-time fluorescence RT-HDA (Reverse Transcriptase-Helicase-Dependent Isothermal Amplification) kit and a primer for detecting avian influenza virus. A group of primers for detecting the avian influenza virus have an oligonucleotides sequence shown by a sequence table SEQ ID No:1 and a sequence table SEQ ID No:2. The kit and the primer have the following advantages that the novel HDA technology is applied to the detection of the avian influenza virus, and compared with the existing AIV (Avian Influenza Virus) detecting method, the HDA detecting method has the advantages of fastness, more convenience, safety and reliability; for the conventional virus separation and serology method, long time is consumed, the bio-safety risk exists; for the HDA method, a natural reproduction mechanism in a body is simulated, the safety is high, the reaction speed is quick, and the detection can be completed by only 2h at most; and compared with fluorescence PCR (Polymerase Chain Reaction), the HDA detecting method has the advantages that the program setting is easier, the reaction conditions such as annealing temperature and the like do not need to be explored, and the HDA detecting method is easier to master and operate in the aspect of technical level. Due to the optimization of reaction conditions, according to the real-time fluorescence RT-HDA method, at least 10copies / reaction of viral nucleic acid can be detected. The technology has a great application prospect in the monitoring and diagnosis of the avian influenza virus, and the kit and the primer are diagnosis tools with good promotion and application values.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Typing detection LAMP primer combination with effect of differentiating porcine parvovirus type 1-7, detection method and kit
ActiveCN110438259AAvoid pollutionSpecific detection methodMicrobiological testing/measurementMicroorganism based processesTypingFluorescence
The invention belongs to the technical field of biology and discloses a typing detection LAMP primer combination with an effect of differentiating porcine parvovirus type 1-7, a detection method and akit. Multiple sets of LAMP primers are designed respectively by utilizing conserved segments of the NS1 gene and NP2 gene of a porcine parvovirus and are screened to obtain the typing detection primer combination which has good specificity and high sensitivity and can be used for differentiating the porcine parvovirus type 1-7. The primer combination can be used for respectively detecting porcineparvovirus type 1-7 and does not have a cross reaction with other porcine viruses. A fluorescent dye is added into a LAMP reaction system, the porcine parvovirus type 1-7 can be detected by a one-step method, whole-process real-time monitoring can be implemented by combining fluorescent quantitation, a result can be obtained within 1 hour, and the detection sensitivity can be 100 copies / [mu]L. The detection method has the advantages of convenience, quickness, sensitiveness and specificity and can avoid an error caused by human determination and environmental pollution caused by cap opening.
Owner:北京市动物疫病预防控制中心 +1
RT-HDA kit and primer for detecting avian influenza virus
ActiveCN102912038BEasy diagnosisEasy to monitorMicrobiological testing/measurementDNA/RNA fragmentationWater bathsSequence ID
The invention discloses an RT-HDA kit for detecting avian influenza virus and a primer. The primer for detecting the avian influenza virus is oligonucleotides sequences shown in a sequence table SEQ ID (Sequence ID) NO: 1 and a sequence table SEQ ID NO:2. According to the RT-HDA kit, a novel constant-temperature amplifying technology HDA is used for detecting the avian influenza virus for the first time; compared with a conventional detection method of AIV (Avian Influenza Virus), the RT-HAD kit has the advantages that: (1) an HDA detection method is faster, more convenient, safer and more reliable. A conventional method of separating virus and a conventional serology method need more time, and bring a risk to safety of an organism; the HDA method simulates a natural replication mechanism in vivo, so that the safety is high, a reaction is fast to carry out, and detection can be accomplished by only two hours; and (2) compared with a PCR (Polymerase Chain Reaction), the HDA detection method has the advantages that PCR instruments are saved, a requirement on equipment is simple, only one water bath kettle is needed, so that the technology is convenient to master and operate, the detection method can be convenient to popularize and use in regions with insufficient conditions, and benefits are provided for diagnosing and monitoring the avian influenza virus.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Real-time fluorescence PCR (polymerase chain reaction) kit and oligonucleotide sequence for detecting swine dysentery
InactiveCN102373276BStrong specificityHigh sensitivityMicrobiological testing/measurementFluorescence/phosphorescencePig farmsNucleotide
The invention discloses a real-time fluorescence PCR (polymerase chain reaction) kit and an oligonucleotide sequence for detecting swine dysentery. The oligonucleotide sequence for detecting swine dysentery is an oligonucleotide sequence shown from SEQ ID No.1 to SEQ ID No.3 in a sequence table. The invention also provides a detection kit containing the oligonucleotide sequence. The kit and the oligonucleotide sequence have the advantages that: when the real-time PCR technology is applied to swine dysentery detection, the detection specificity and sensitivity are further improved, the workload is reduced, the working efficiency is improved, and a target fragment can be quantitatively detected. By virtue of optimization of reaction conditions, the real-time PCR can detect pathogen amount of 10<2> copy / reaction minimally. 318 clinical samples from 7 pig farms are detected by using the method, and experiment results prove that: the method is a specific, sensitive and efficient detection method. The technology has great application prospect in import and export sample quarantine, as well as monitor and diagnosis of clinical plague.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU +2
Real-time fluorescence PCR (polymerase chain reaction) kit and oligonucleotide sequence for detecting swine dysentery
InactiveCN102373276AStrong specificityHigh sensitivityMicrobiological testing/measurementFluorescence/phosphorescencePig farmsNucleotide
The invention discloses a real-time fluorescence PCR (polymerase chain reaction) kit and an oligonucleotide sequence for detecting swine dysentery. The oligonucleotide sequence for detecting swine dysentery is an oligonucleotide sequence shown from SEQ ID No.1 to SEQ ID No.3 in a sequence table. The invention also provides a detection kit containing the oligonucleotide sequence. The kit and the oligonucleotide sequence have the advantages that: when the real-time PCR technology is applied to swine dysentery detection, the detection specificity and sensitivity are further improved, the workload is reduced, the working efficiency is improved, and a target fragment can be quantitatively detected. By virtue of optimization of reaction conditions, the real-time PCR can detect pathogen amount of 10<2> copy / reaction minimally. 318 clinical samples from 7 pig farms are detected by using the method, and experiment results prove that: the method is a specific, sensitive and efficient detection method. The technology has great application prospect in import and export sample quarantine, as well as monitor and diagnosis of clinical plague.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU +2
Sucrose-glucose-fructose content determination kit and determination method thereof
PendingCN110923113AThe test result is accurateShorten experimental operation timeBioreactor/fermenter combinationsBiological substance pretreatmentsFructoseSucrose
The invention relates to the technical field of sugar content determination, and discloses a sucrose-glucose-fructose content determination kit and a determination method thereof. The sucrose-glucose-fructose content determination kit comprises a kit body, a reagent tube, a movable connecting device, a sealing cover and a locking device, wherein the reagent tube is fixedly arranged in the kit body; the movable connecting device is fixedly arranged on the right side of the kit body; and the sealing cover is fixedly arranged at the right end of the movable connecting device. According to the sucrose-glucose-fructose content determination kit and the determination method thereof, specific enzymes respectively act on sucrose, glucose and fructose, background interference of other substances ina sample is eliminated, and the detection result is accurate; and moreover, the three pieces of sugar data can be detected at one time, the experimental operation time is shortened, personal errors caused by batch detection are reduced, and a time-saving, money-saving and nontoxic detection means is provided for detection of a large number of samples.
Owner:苏州格锐思生物科技有限公司
Fluorescent PCR detection reagent for identifying bovine gene source in heparin, preparation method and application
ActiveCN103937881BHigh sensitivityWide linear rangeMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceQuantitative determination
The invention discloses a fluorescent PCR detection reagent for identifying cattle gene source in heparin, a preparation method and application, provides a detection method for detecting cattle composition source in a heparin crude finished product, a real-time fluorescence quantification PCR primer and a probe by performing sequencing and comparison on cattle gene, and the length of an amplification target fragment for cattle composition detection is 92 bp. The invention also provides a kit for performing quantitative determination on cattle gene. The detection method and the detection kit have the advantages of accurate detection, high sensitivity, strong specificity and the like, and have good specimen detection capability.
Owner:BEIJING POLYTECHNIC
Monoclonal antibody against riemerella anatipestifer (RA)
InactiveCN102304493BRapid diagnosisSpecific diagnosisMicroorganism based processesTissue cultureMedicineMonoclonal antibody
The invention belongs to the field of biotechnology, specifically relates to a monoclonal antibody against riemerella anatipestifer (RA). The preservation number of the monoclonal antibody 5G7 is CGMCC No. 5165. With adopting the monoclonal antibody to prepare the immune colloidal gold strip for detecting the RA, the pathogen of the RA can be directly detected, and the RA strains such as serotypes of 1, 2, 3 and 11 can be synchronously detected, such that the monoclonal antibody against the RA has a wide application value.
Owner:YANGZHOU UNIV
A primer pair for preparing and detecting avian adenovirus type 4 kit and its application
ActiveCN105886502BHigh detection sensitivityThe result is accurateMicrobiological testing/measurementDNA/RNA fragmentationDiseaseNucleotide
The invention discloses a primer pair for preparing a kit for detecting type-4 avian adenovirus and an application thereof. The primer pair comprises an upstream primer and a downstream primer, wherein the nucleotide sequence of the upstream primer is shown by SEQ ID No.1; and the nucleotide sequence of the downstream primer is shown by SEQ ID No.2. The invention also discloses a kit comprising the primer pair and a method for detecting the type-4 avian adenovirus. The primer pair can specifically amplify the Hexon gene segment of the type-4 avian adenovirus in a sample, the detection sensitivity is high, the lowest detection limit reaches 7.2*10<4>ng / mu L, the result is accurate, and the primer pair can be applied to the detection and identification of the type-4 avian adenovirus; the primer pair is universal in detecting different strains of the type-4 avian adenovirus; and the rapid, specific and accurate detection method of the type-4 avian adenovirus provides a detection tool for detecting and controlling the prevalence of the disease.
Owner:ZHEJIANG UNIV
RT-HDA (Reverse Transcription-Helicase-Dependent Isothermal Amplification) kit and primer for detecting foot-and-mouth disease virus
ActiveCN102912039BEasy diagnosisEasy to monitorMicrobiological testing/measurementDNA/RNA fragmentationWater bathsHelicase
The invention discloses an RT-HDA (Reverse Transcription-Helicase-Dependent Isothermal Amplification) kit and primers for detecting a foot-and-mouth disease virus. The s for detecting a foot-and-mouth disease virus are oligonucleotide sequences shown in the sequence list SEQ ID No:1 and the sequence table SEQ ID No:2. In the invention, the novel isothermal amplification technology HDA is applied to the detection of the foot-and-mouth disease virus for the first time. Compared with the traditional FDMV detection method, the HDA detection method is quicker, more convenient, safer and more reliable. The traditional virus isolation and serological method has long time consumption and biosafety risk. The HDA method imitates the natural replication mechanism in the body and has high safety and quick response, and only 2 h are needed at most to complete detection. Compared with PCR (Polymerase Chain Reaction), the HDA method does not need any PCR instrument, the equipment requirement is simple, only one water bath kettle is needed, and the HDA method is easier to master and operate speaking from the technology level, is easier to be popularized and applied to regions with insufficient conditions and is more conducive to the diagnosis and monitoring of the foot-and-mouth disease virus.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Pythium splendens braun fluorescence quantitative PCR detection reagent and detection kit and application
InactiveCN102382890BAccurate detectionSensitiveMicrobiological testing/measurementFluorescence/phosphorescenceElectrophoresesTotal rna
The invention discloses a pythium splendens braun fluorescence quantitative PCR detection reagent, a detection kit and an application; the detection reagent comprises a pair of specific primers with sequences as shown in SEQ ID NO: 1 and SEQ ID NO: 2, and a specific fluorescence probe with a sequence as shown in SEQ ID NO: 3; an amplification target fragment has a length of 94 bp, and the nucleotide sequence of the amplification target fragment is shown in SEQ ID NO: 4; the detection kit comprises components of a CTAB extract, a PCR buffer containing the primers and the probe, TaqDNA polymerase, positive control liquid, and quantitative standard liquid; the detection method comprises the extraction of total RNA and the fluorescence PCR reaction; detection performed by using the pythium splendens braun fluorescence quantitative PCR detection reagent overcomes disadvantages of time consumption, easy pollution, and electrophoresis detection necessity after amplification of routine PCR, can realize rapid and accurate qualitative and quantitative detection of pythium splendens braun in a sample, and has the advantages of simplicity, easy operations, intuitive results, high sensitivity, good repeatability, and the like.
Owner:NINGBO ACAD OF SCI & TECH FOR INSPECTION & QUARANTINE
Primer for discriminating schistosoma japonicas, corresponding kit and detection method thereof
InactiveCN102021247BSpecific detection methodFast detection methodMicrobiological testing/measurementDNA/RNA fragmentationBiotechnologyBiochemistry
The invention discloses a primer for discriminating schistosoma japonicas, a corresponding kit and a detection method thereof. The sequence of an upstream primer for discriminating the schistosoma japonicas is shown as SEQIDNO:1, and the sequence of a downstream primer is shown as SEQIDNO:2. In the invention, a method for fast detecting the schistosoma japonica is also established, and the schistosoma japonicas with different types can be discriminated accurately. The primer disclosed by the invention is prepared into the kit, has the advantages that the operating procedure is simplified, the detecting specificity is strong, the sensitivity is high and the result judgment is objective and the like and is suitable for being promoted and applied.
Owner:SOUTH CHINA AGRI UNIV
PCR (Polymerase Chain Reaction) kit and primers for detecting swine dysentery
InactiveCN102373277AEasy diagnosisEasy to monitorMicrobiological testing/measurementDNA/RNA fragmentationNucleotideNucleotide sequencing
The invention discloses a PCR (Polymerase Chain Reaction) kit and primers for detecting swine dysentery. A group of primers for detecting the swine dysentery have the nucleotide sequences disclosed in a sequence table SEQIDNo: 1 and a sequence table SEQIDNo: 2. The invention has the advantages that compared with etiology separation culture, the PCR technology applied in the detection of the swinedysentery is faster and more convenient, the bacterium separation culture method at least needs 10 days and hard culture conditions, strict anaerobic culture is needed, and the PCR can be completed only in 5 hours; and compared with etiology separation, the PCR needs relatively simple equipment, is more convenient to master and operate from a technical perspective, is more convenient to apply andpopularize in areas with insufficient conditions and is more conductive to the diagnosis and monitoring of the swine dysentery.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU +2
Aeromonas hydrophila tetracycline drug-resistance gene detection method
InactiveCN104878081ASensitive detection methodSpecific detection methodMicrobiological testing/measurementMicroorganism based processesDrugAeromonas hydrophila
The invention discloses an aeromonas hydrophila tetracycline drug-resistance gene detection method. The method comprises the following steps: (1) collecting 0.5-0.7 g of aeromonas hydrophila living bacteria, resuspending with 5-7 mL of deionized water, repeatedly freeze-thawing to break cells, centrifuging and collecting a supernatant to obtain a template; (2) mixing the template with tetracycline drug-resistance gene detection primers TetA-F, TetA-R, TetC-F, TetC-R, TetG-F and TetG-R, and carrying out PCR amplification; and (3) carrying out agarose gel electrophoresis after PCR amplification in the step (2) and sending for sequencing. The detection method provided by the invention is sensitive, specific and rapid and can be adopted to detect a trace amount of target gene without the time-consuming aeromonas hydrophila culture stage.
Owner:MINNAN NORMAL UNIV
Real-time fluorescence RT-HDA (Reverse Transcriptase-Helicase-Dependent Isothermal Amplification) kit and primer for detecting foot-and-mouth disease virus
ActiveCN102876814BFast detection methodThe detection method is convenient and fastMicrobiological testing/measurementFluorescence/phosphorescenceReverse transcriptaseFluorescence
The invention discloses a real-time fluorescence RT-HDA (Reverse Transcriptase-Helicase-Dependent Isothermal Amplification) kit and primer for detecting foot-and-mouth disease virus. A group of primers for detecting the foot-and-mouth disease virus have an oligonucleotides sequence shown by a sequence table SEQ ID No:1 and a sequence table SEQ ID No:2. The kit and the primer have the following advantages that the novel HAD technology is applied to the detection of the foot-and-mouth disease virus, and compared with the existing FMDV (Foot-And-Mouth Disease Virus) detecting method, the HDA detecting method has the advantages of fastness, more convenience, safety and reliability; for the conventional virus separation and serology method, long time is consumed, the bio-safety risk exists; for the HDA method, a natural reproduction mechanism in a body is simulated, the safety is high, the reaction speed is quick, and the detection can be completed by only 2 hours at most; and compared with fluorescence PCR (Polymerase Chain Reaction), the HDA detecting method has the advantages that the program setting is easier, the reaction conditions such as annealing temperature and the like do not need to be explored, and the HDA detecting method is easier to master and operate in the aspect of technical level. Due to the optimization of reaction conditions, according to the real-time fluorescence RT-HDA method, at least 10copies / reaction of viral nucleic acid can be detected. The technology has a great application prospect in the monitoring and diagnosis of the foot-and-mouth disease virus, and the kit and the primer are diagnosis tools with good promotion and application values.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Detection method and culture medium of Bacillus coagulans
ActiveCN108034607BRich detection methodsSpecific detection methodBacteriaMicrobiological testing/measurementBiotechnologyYeast cell extract
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com