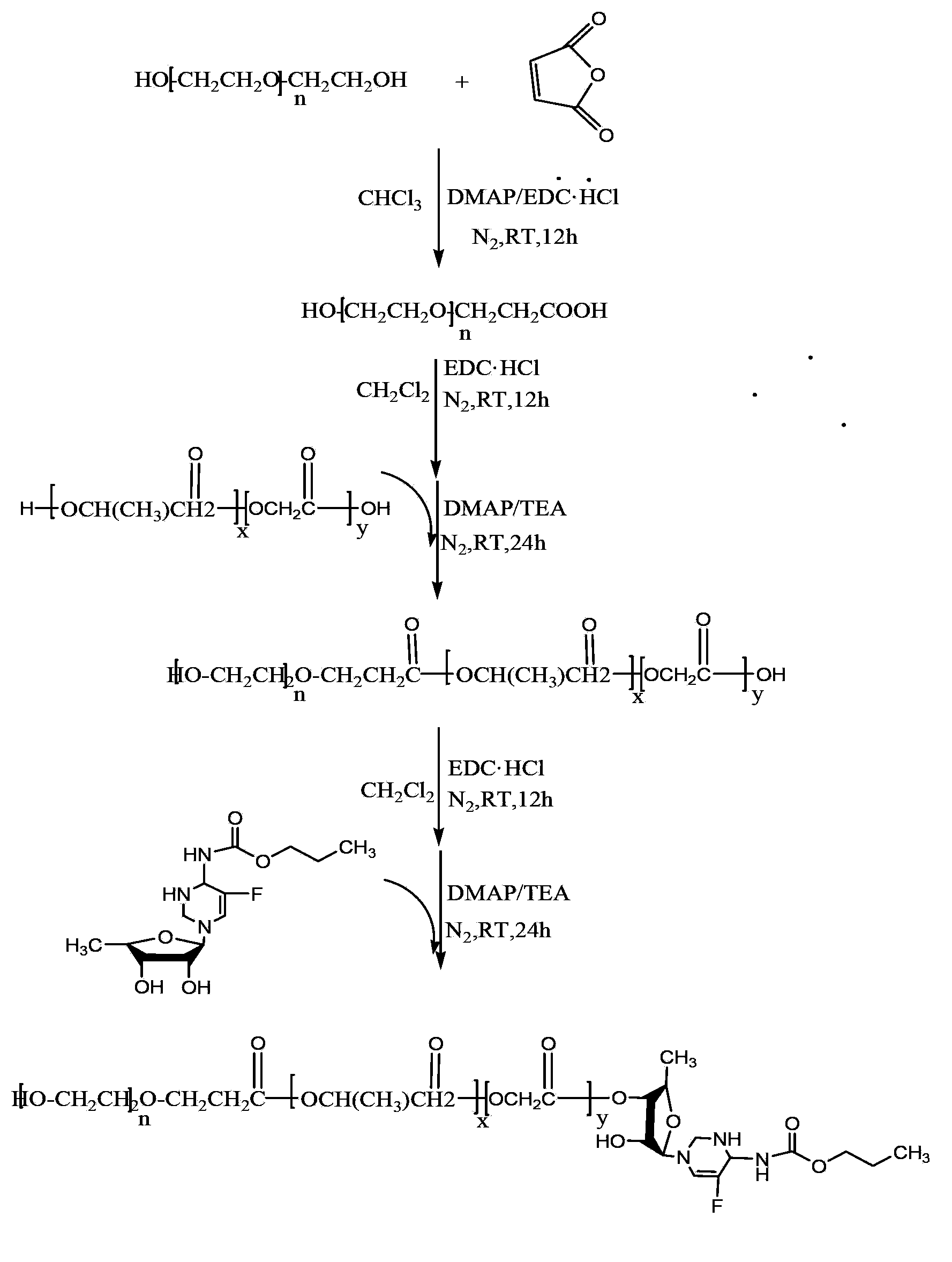
Capecitabine medicine carrier and preparation method thereof
A drug and carrier technology, applied in the field of capecitabine drug carrier and its preparation, can solve the problems of normal cell damage, frequent drug use, and long duration of the process, so as to maintain the activity of the drug, increase the circulation time in the body, and improve the utilization rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Synthesis of carboxyl-terminated polyethylene glycol
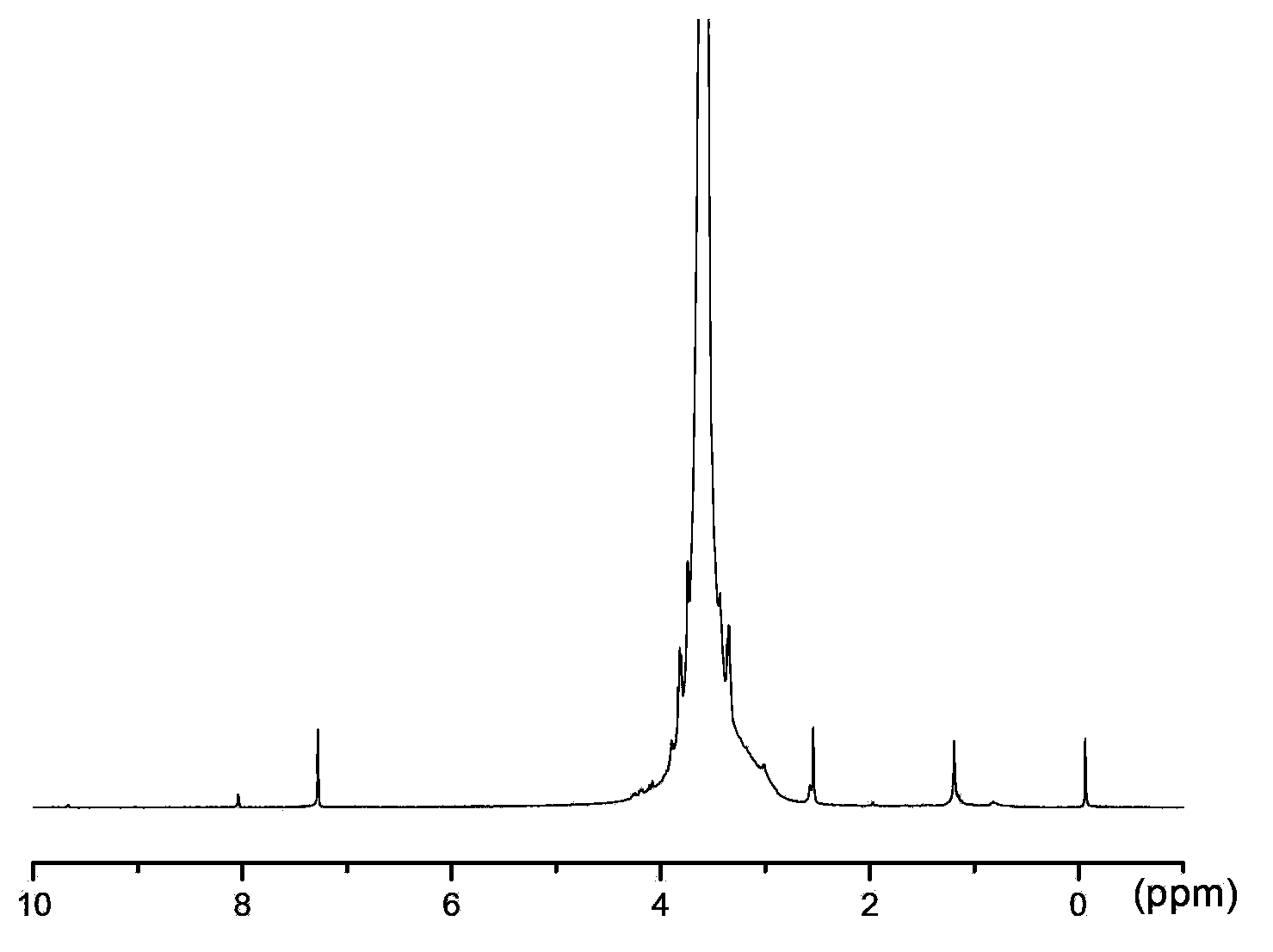
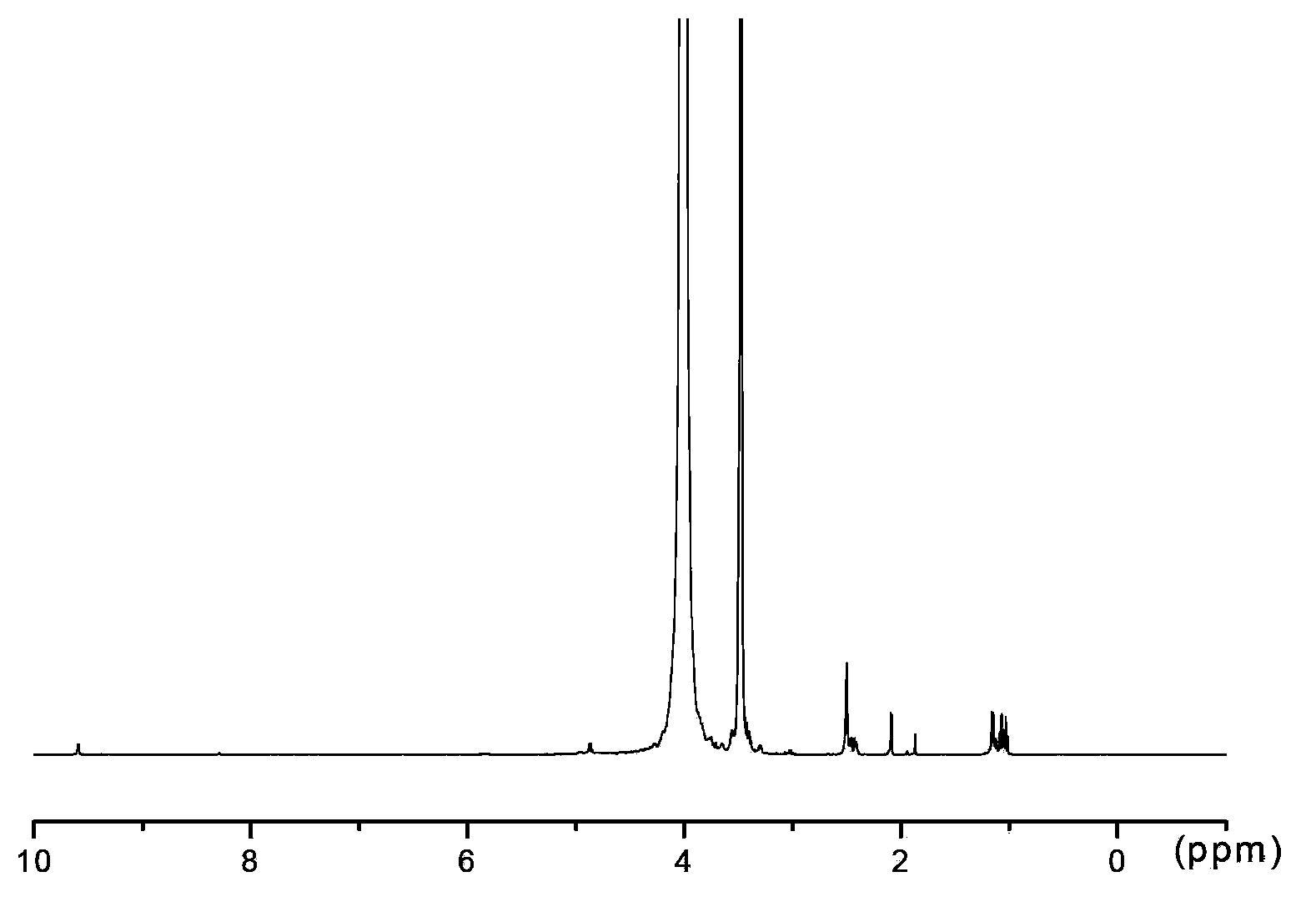
[0047] Dissolve 10.000g of polyethylene glycol (molecular mass: 2000Da), 0.502g of succinic anhydride in 30mL of dry chloroform, then add 1.151g of EDC·HCl, 0.122g of DMAP and 51uL of triethylamine. Stir the reaction at room temperature for 12h, then rotevaporate at 52°C to remove chloroform to obtain a residue; dissolve the residue in 35mL saturated NaHCO 3 , remove the white insoluble matter by suction filtration under reduced pressure; take the filtrate and wash it with 20mLCHCl 3 Extract the filtrate 3 times, take the water phase, adjust the pH to 2 with 0.1mol / L HCl, then transfer to a separatory funnel, add 20mL CHCl 3 Extract 3 times, combine extracts, add 1.200g anhydrous Na 2 SO 4 Dry until clarified, filter, take the filtrate, concentrate to about 3mL by rotary evaporation at 52°C, add 60mL of anhydrous ether to crystallize, put in the refrigerator at -4°C for 24 hours, filter, and vacuum dry at 25°...
Embodiment 2
[0073] (1) Synthesis of carboxyl-terminated polyethylene glycol
[0074] Dissolve 10.000g of polyethylene glycol (molecular mass: 4000Da), 0.625g of succinic anhydride in 30mL of dry chloroform, then add 0.575g of EDC·HCl, 0.061g of DMAP and 26uL of triethylamine. Stir the reaction at room temperature for 12h, then rotevaporate at 52°C to remove chloroform to obtain a residue; dissolve the residue in 35mL saturated NaHCO 3 , remove the white insoluble matter by suction filtration under reduced pressure, and take the filtrate; use 20mLCHCl 3 Extract the filtrate 3 times, take the water phase, adjust the pH to 2 with 0.1mol / L HCl, then transfer to a separatory funnel, add 20mL CHCl 3 Extract 3 times, combine extracts, add 1.200g anhydrous Na 2 SO 4 Dry until clarified, filter, take the filtrate, concentrate to about 3mL by rotary evaporation at 52°C, add 60mL of anhydrous ether to crystallize, put in the refrigerator at -4°C for 24 hours, filter, and vacuum dry at 25°C to obt...
Embodiment 3
[0099] (1) Synthesis of carboxyl-terminated polyethylene glycol
[0100] Dissolve 10.000g of polyethylene glycol (molecular mass: 10000Da), 0.301g of succinic anhydride in 30mL of dry chloroform, then add 0.288g of EDC·HCl, 0.122g of DMAP and 50uL of triethylamine. The reaction was stirred at room temperature for 12 h, then 53 ° C rotary evaporation, chloroform was removed to obtain a residue; the residue was dissolved in 25 mL saturated NaHCO 3 , remove the white insoluble matter by suction filtration under reduced pressure, and take the filtrate; use 20mLCHCl 3 Extract the filtrate 3 times, take the water phase, adjust the pH to 2 with 0.1mol / L HCl, then transfer to a separatory funnel, add 20mL CHCl 3 Extract 3 times, combine extracts, add 5g anhydrous Na 2 SO 4 Dry until clarified, filter, take the filtrate, concentrate to about 3mL by rotary evaporation at 53°C, add 60mL of anhydrous ether to crystallize, put in the refrigerator at -4°C for 24 hours, filter, and vacuum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com