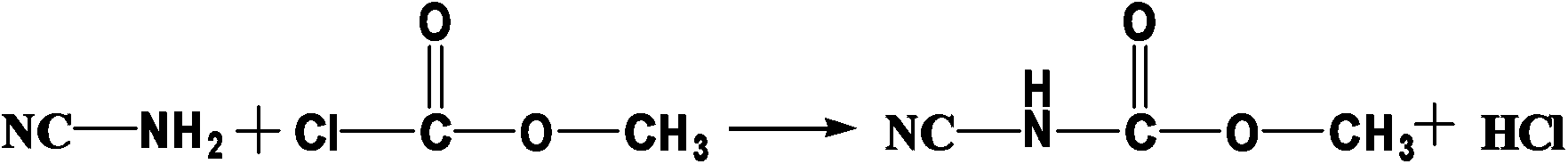
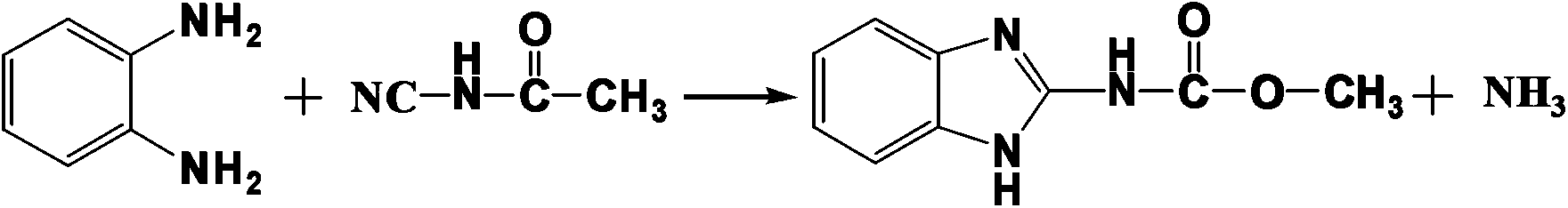
Synthetic method of N-(2-benzimidazolyl)-methyl carbamate
A technology of methyl carbamate and benzimidazolyl, applied in the field of organic synthesis, can solve the problems of serious environmental pollution, difficult operation, polluted environment and the like, and achieves the effects of high yield, simple operation and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A kind of synthetic method of N-(2-benzimidazolyl)-carbamic acid methyl ester, its concrete steps are:
[0023] (1) 11.5g of methyl chloroformate and 16.4g of 50% sodium phosphate aqueous solution were added dropwise to 21.0g of 30% cyanamide aqueous solution at the same time. During the dropwise addition, the pH value of the reaction was controlled to be 7.0, and the reaction temperature was controlled at 5°C. After completion, the reaction was incubated for 2 hours;
[0024] (2) The above reaction solution was heated to 95°C, 10.8 g of o-phenylenediamine was put into it, and the reaction was kept for 3 hours. The obtained product was detected by high performance liquid chromatography (methanol:water=60:40, wavelength 230nm chromatographic column 20RBAX SB-C184.6*150mm5μm, flow rate 1.0ml / min, column temperature 30℃). The yield is 90.6%, and the content is 98.0%.
Embodiment 2
[0026] A kind of synthetic method of N-(2-benzimidazolyl)-carbamic acid methyl ester, its concrete steps are:
[0027] (1) 14.2g of methyl chloroformate and 11.2g of 50% potassium carbonate aqueous solution were added dropwise to 12.8g of 40% cyanamide aqueous solution. During the dropwise addition, the pH value of the reaction was controlled to be 7.2, and the reaction temperature was controlled at 10°C. After completion, the insulation reaction was carried out for 2.3 hours;
[0028] (2) The above reaction solution was heated to 93°C, 10.8g of o-phenylenediamine was put into it, and the reaction was incubated for 3.5 hours. The obtained product was detected by high performance liquid chromatography (methanol:water=60:40, wavelength 230nm chromatographic column 20RBAX SB-C184.6*150mm5μm, flow rate 1.0ml / min, column temperature 30℃). The yield is 89.5%, and the content is 98.1%.
Embodiment 3
[0030] A kind of synthetic method of N-(2-benzimidazolyl)-carbamic acid methyl ester, its concrete steps are:
[0031] (1) 14.2g of methyl chloroformate and 11.4g of 50% sodium phosphate aqueous solution were added dropwise to 14.0g of 30% cyanamide aqueous solution respectively. During the dropwise addition, the pH value of the reaction was controlled to be 7.4, and the reaction temperature was controlled at 8°C. After completion, the insulation reaction was carried out for 2.4 hours;
[0032] (2) The above reaction solution was heated to 91°C, 10.8g of o-phenylenediamine was put into it, and the reaction was kept for 3.2 hours. The obtained product was detected by high performance liquid chromatography (methanol:water=60:40, wavelength 230nm chromatographic column 20RBAX SB-C184.6*150mm5μm, flow rate 1.0ml / min, column temperature 30℃). The yield is 90.3%, and the content is 98.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com