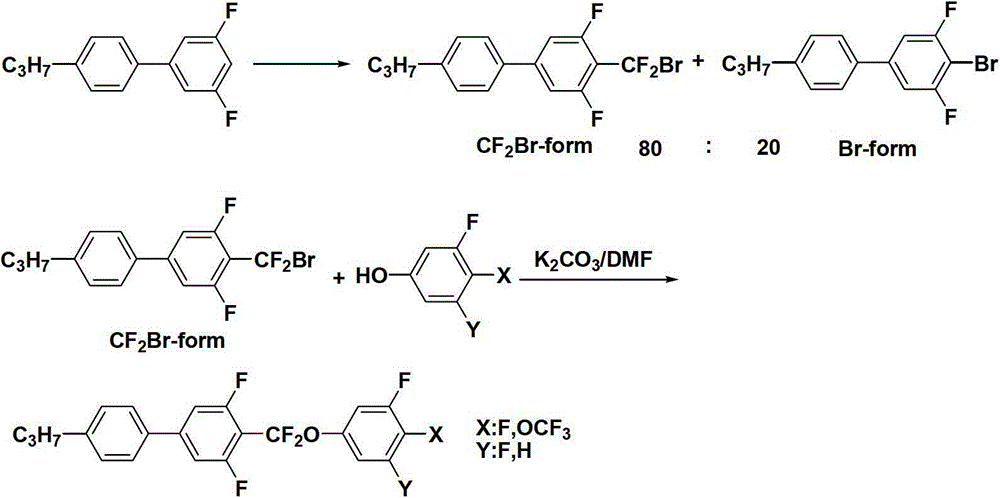
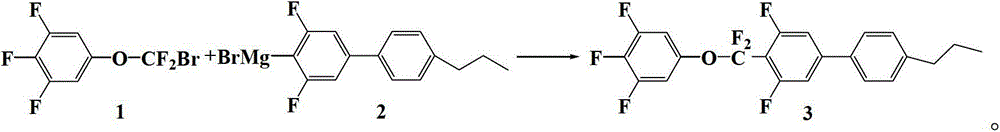
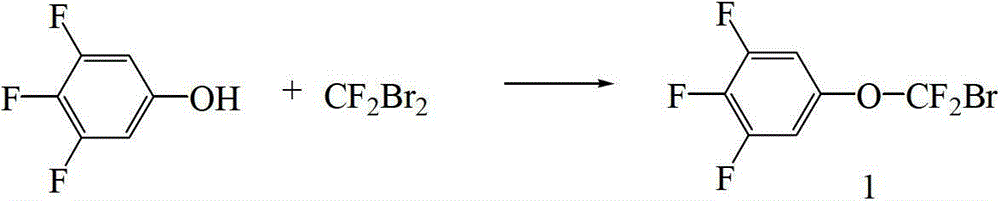Preparation method of CF2O-containing compound, and intermediate compound and preparation method thereof
A compound and catalyst technology, applied in the field of preparation of CF2O-containing compounds, intermediates and preparation thereof, can solve the problems of unfavorable scale-up production, low product yield and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0075] The preparation of embodiment 14-bromo-3,5-difluoro-4'-propylbiphenyl
[0076]Add 420g of tetrahydrofuran (THF) and 185g (0.80mol) of 3,5-difluoro-4'-propylbiphenyl into a 2000mL four-neck flask, replace with nitrogen for 3 times, and cool down to -50 after the replacement is completed. ℃, after cooling down, slowly add 400mL (1.00mol, 2.5mol / L) of n-butyllithium n-hexane solution dropwise. After the addition, the temperature was kept for 2 hours, and after the heat preservation was completed, the temperature was naturally raised to room temperature. Add 365g of an aqueous solution of hydrochloric acid (the mass percent refers to the percentage of the mass of hydrogen chloride in the total mass of the aqueous solution of hydrochloric acid) with a mass percentage of 10%, evaporate the solvent, then extract with 200g×3 petroleum ether, and combine the organic layers. Add 50 g of anhydrous magnesium sulfate, stir for 10 minutes, pass through a 40 g silica gel column, and ...
Embodiment 24
[0077] Preparation of embodiment 24-bromo-3,5-difluoro-4'-propylbiphenyl Grignard reagent
[0078] Add 21.2g (0.88mol) of magnesium chips, 0.0227g of iodine and 200g of tetrahydrofuran (THF) into a 1000mL four-neck flask, replace with nitrogen three times, stir at 20°C, and dropwise add 4-bromo-3,5-bis Fluoro-4'-propyl biphenyl crude product 23.8g (0.074mol) and tetrahydrofuran (THF) 100g mixed solution. Stir after the dropwise addition, and after the color of the solution fades, add dropwise a mixture of 212.6g (0.661mol) of crude 4-bromo-3,5-difluoro-4'-propylbiphenyl and 200g tetrahydrofuran (THF) at 20°C . After the dropwise addition, the temperature was kept, and the dropwise addition time and the heat preservation time were 4 hours in total. Then 4-bromo-3,5-difluoro-4'-propylbiphenyl Grignard reagent is obtained. It was directly used in the next reaction without purification.
Embodiment 35
[0079] Example 35- Preparation of (bromo-difluoro-methoxy)-1,2,3-trifluorobenzene
[0080] Add 600 g of N,N-dimethylacetamide (DMAC) into a 2000 mL four-necked bottle. At 20°C, 11.5 g (0.5 mol) of sodium and 4.8 g (0.2 mol) of sodium hydride were sequentially added. After stirring for 1 hour, start to add dropwise a mixture of 200 g (1.35 mol) of 3,4,5-trifluorophenol and 200 g of N,N-dimethylacetamide (DMAC). After the dropwise addition, keep stirring for 2 hours. Start to feed 337g (1.62mol) of difluorodibromomethane. After the dropwise addition, keep stirring for 6 hours. After keeping warm, pour the reaction solution into a 5000mL glass reactor, add 1000g methyl tert-butyl ether and 2000g water, stir, and divide After the solution was extracted with 200g×2 methyl tert-butyl ether, the combined organic layers were washed with 200g×3 water, and after washing, the methyl tert-butyl ether was evaporated (methyl tert-butyl ether can be recycled and used mechanically), and petr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com