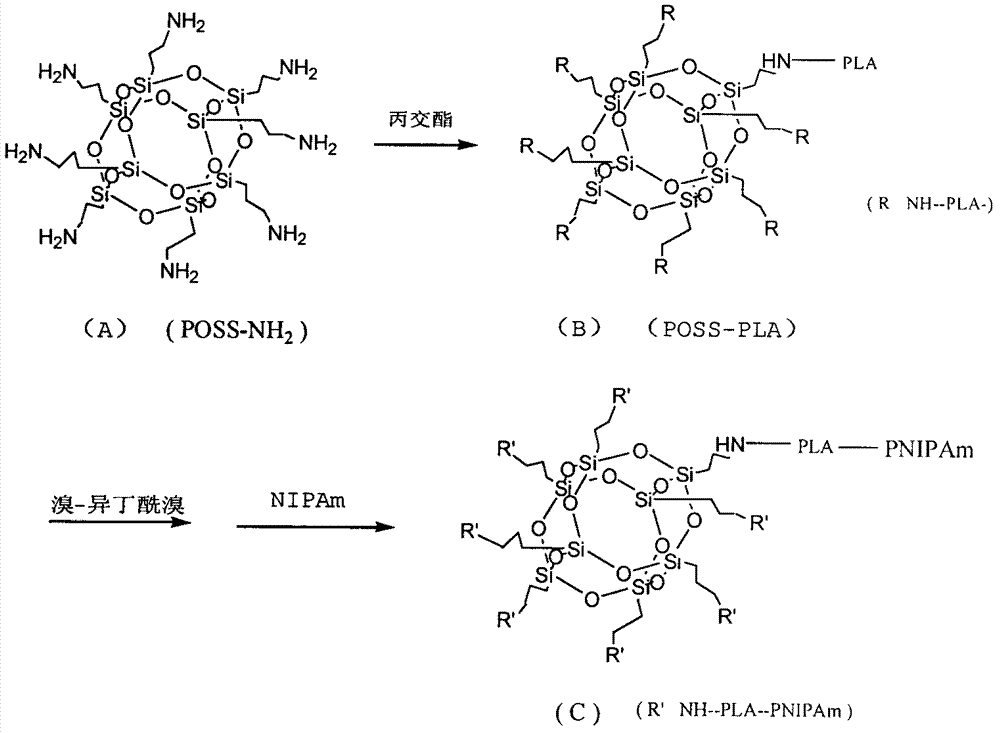
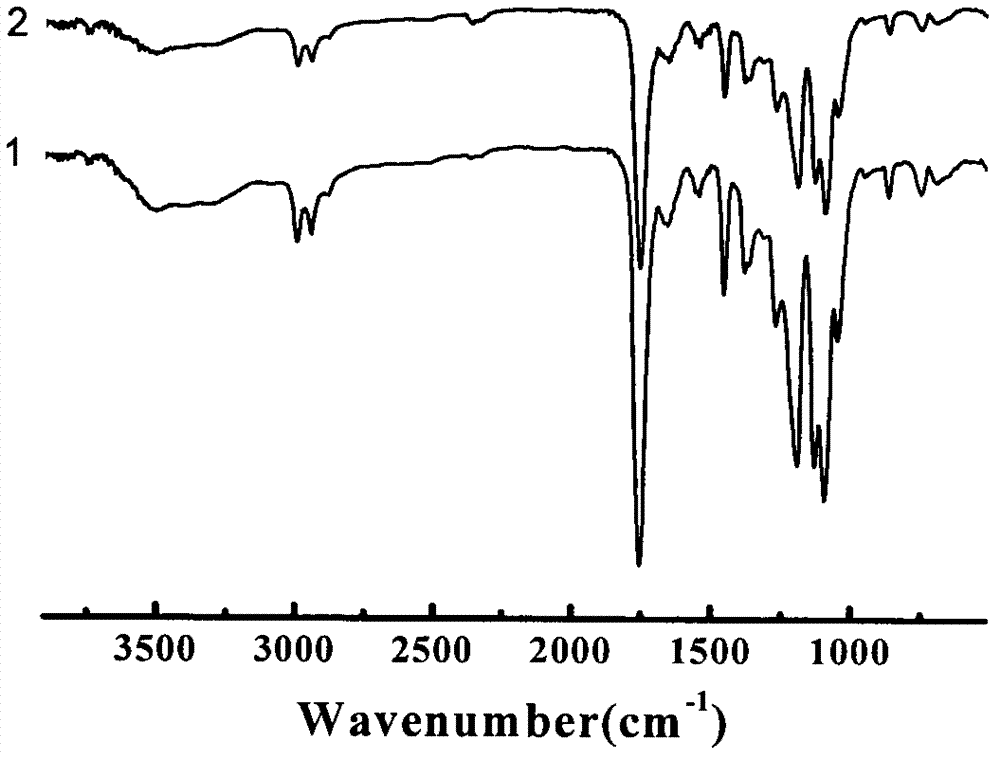
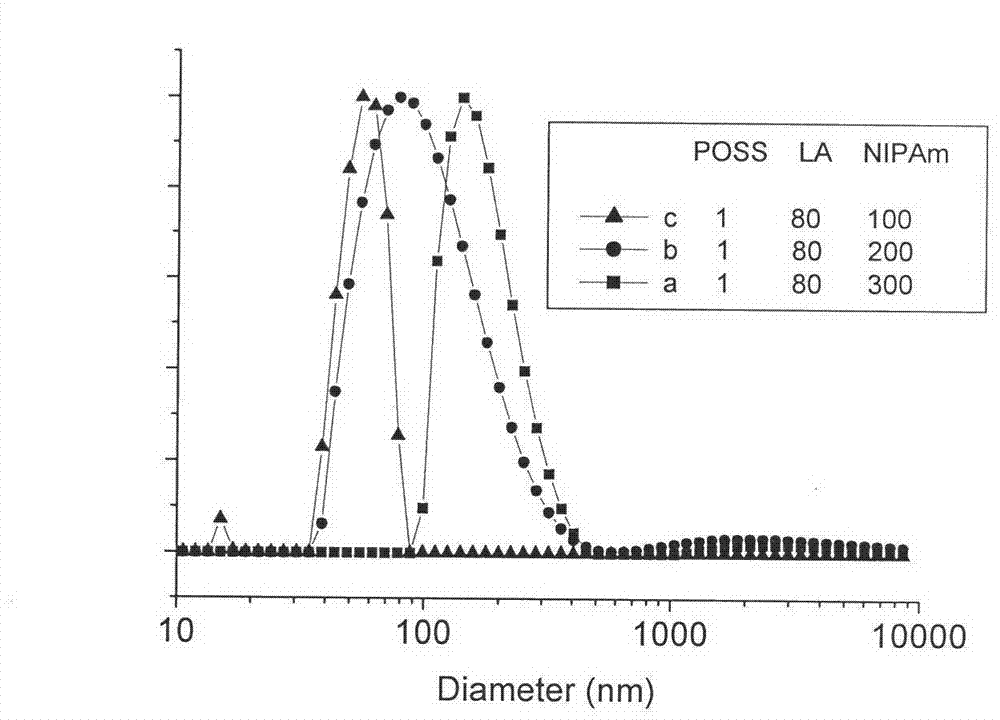
POSS/poly lactic acid and poly(n-isopropyl acrylamide) star block copolymer preparation
A technology of block copolymers and polylactic acid, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, emulsion delivery, etc., can solve the problem of dissociation of micelles, irregular micellar structure, Changeable shape and other problems, to achieve the effect of controllable size, stable product structure and regular shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 Alkali-catalyzed synthesis of primary amino cage silsesquioxane:
[0024] Add 23 mL of deionized water, 13 mL of propanol, 3 mL of acetonitrile and 0.5 mL of tetraethylammonium hydroxide aqueous solution into a 100 mL flask and mix, and mix 55 g of γ-aminopropyltriethoxysilane under vigorous stirring in Add to the reaction system within 10 minutes, heat the solution to 55°C under vigorous stirring, stop after 20 hours of reaction, add about 2 times the volume of tetrahydrofuran to the reaction solution, a white precipitate is formed, collect the white precipitate, and dry .
Embodiment 2
[0025] Embodiment 2POSS-(NH 2 ) 8 Initiate the living ring-opening polymerization of D,L-lactide:
[0026] Weigh POSS-(NH 2 ) 8 1.762 grams (0.002mol), D, L-lactide 11.52 grams (0.080mol), placed in a 50ml single-necked flask, vacuum-filled with nitrogen for three times, stirred and melted in an oil bath at 135°C for 5min, then added D with a syringe , 0.5% stannous octoate-toluene solution of L-lactide quality, then vacuum-filled nitrogen cycle three times, reacted at 135 ° C for 24 hours. Natural cooling after stopping the reaction, the product was dissolved with 5 mL of dichloromethane, and then A large amount of n-hexane precipitated and repeated three times to obtain a light yellow liquid, which was vacuum-dried to obtain POSS-PLA-1, a star-shaped polymer with regular structure.
Embodiment 3
[0028] Weigh POSS-(NH 2 ) 8 1.762 grams (0.002mol), D, 23.04 grams (0.16mol) of L-lactide, other materials and operation methods are reacted as embodiment 2, obtain POSS-PLA-2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com