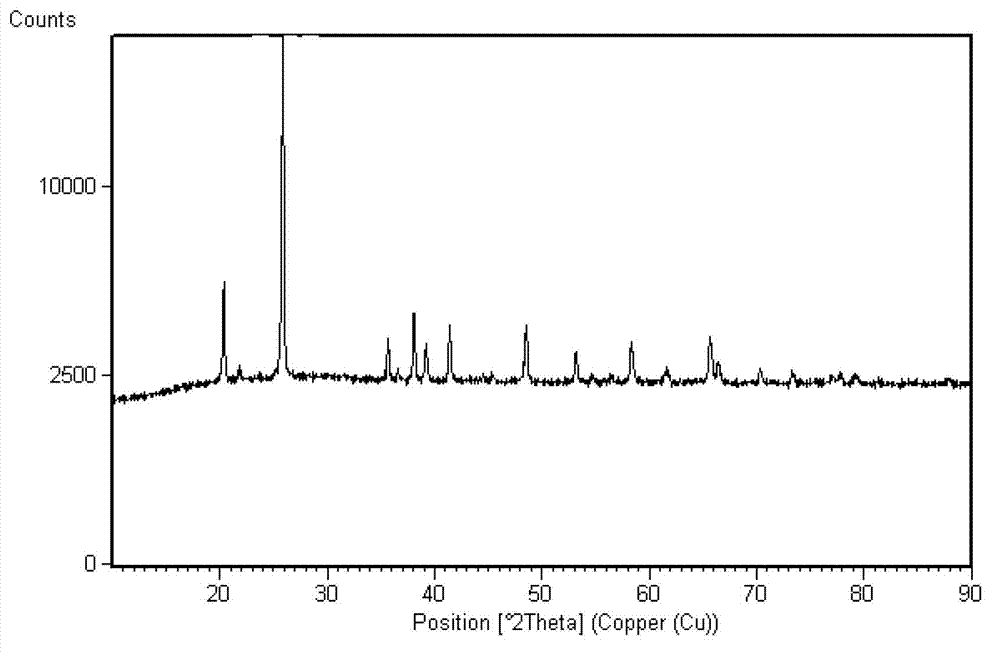
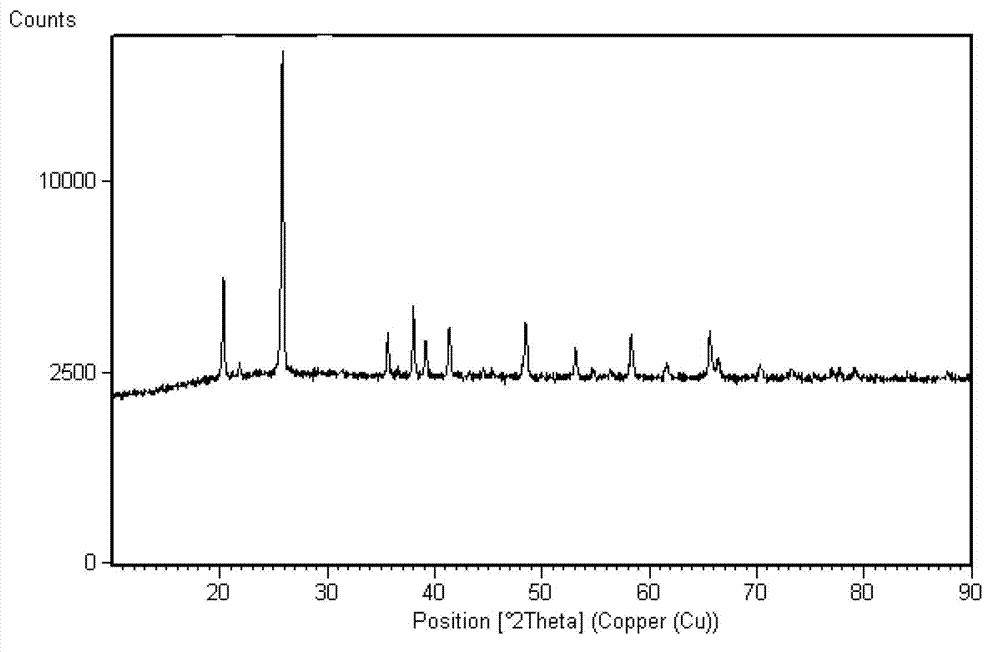
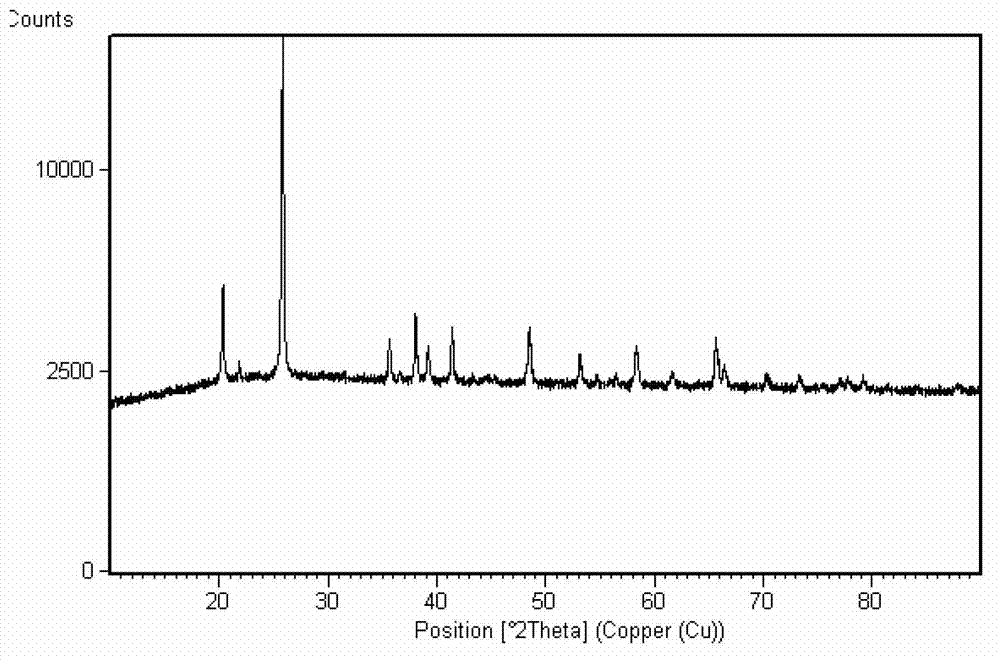
Method for preparing ferric pyrophosphate for lithium battery and ferric pyrophosphate prepared by method
A technology of iron orthophosphate and lithium batteries, which is applied in the direction of phosphate, battery electrodes, phosphorus oxyacids, etc., can solve the problems of high cost, achieve the effects of ensuring utilization rate, reducing production cost, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The preparation method of ferric orthophosphate disclosed by the invention comprises:
[0023] S1, pulverizing ferrophosphorus raw materials to obtain ferrophosphorus fines;
[0024] S2. Mix the ferrophosphorus fine material with the acid solution, then pass through the oxidizing gas, and filter to obtain solution A; wherein, the acid solution is selected from one or more of sulfuric acid, hydrochloric acid or nitric acid;
[0025] S3, adding a phosphorus source to solution A to obtain solution B; the phosphorus source is selected from phosphoric acid and / or phosphate;
[0026] S4, in the stirring state, add solution B and lye into the container in parallel, and control the pH value of the solution in the container to remain at 2.0-2.5, continue stirring to obtain a slurry with ferric orthophosphate;
[0027] S5. Separating the solid phase and mother liquor in the slurry, washing and drying the solid phase to obtain ferric orthophosphate.
[0028] The invention adopts...
Embodiment 1
[0055] This example is used to illustrate the preparation method of the ferric orthophosphate disclosed by the present invention.
[0056] Weigh 1000 g of ferrophosphorus slag from the yellow phosphorus electric furnace, and crush it to 60 mesh with a jaw crusher to obtain ferrophosphorus fines.
[0057] Determine that wherein the massfraction of iron is 72.5%, the massfraction of phosphorus is 23.4%, the massfraction of silicon is 1.8%, the massfraction of manganese is 1.1%, the massfraction of nickel is 0.8%, and the massfraction of titanium is determined by ICP test. 0.1%, the mass fraction of carbon is 0.1%, the mass fraction of calcium is 0.09%, and the mass fraction of sulfur is 0.05%.
[0058] Add 5000g of deionized water into a 10L reactor lined with a PE jacketed electric heater, weigh 500g of the above-mentioned ferrophosphorus fine material, add it to the reactor and stir, add 800g of industrial concentrated solution with a mass fraction of 98% to the reactor. Sulf...
Embodiment 2
[0063] This example is used to illustrate the preparation method of the ferric orthophosphate disclosed by the present invention.
[0064] Weigh 1000 g of ferrophosphorus slag from the yellow phosphorus electric furnace, and crush it to 60 mesh with a jaw crusher to obtain ferrophosphorus fines.
[0065] Determine that wherein the massfraction of iron is 74.3%, the massfraction of phosphorus is 22.1%, the massfraction of silicon is 1.5%, the massfraction of manganese is 1.3%, the massfraction of nickel is 0.4%, and the massfraction of titanium is determined by ICP test. 0.2%, the mass fraction of carbon is 0.06%, the mass fraction of calcium is 0.03%, and the mass fraction of sulfur is 0.03%.
[0066] Add 5000g of deionized water to a 10L reactor lined with a PE jacketed electric heater, weigh 500g of the above ferrophosphorus fines, add to the reactor and stir, add 1600g of industrial hydrochloric acid with a mass fraction of 36.5% to the reactor , turn on the electric heate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com