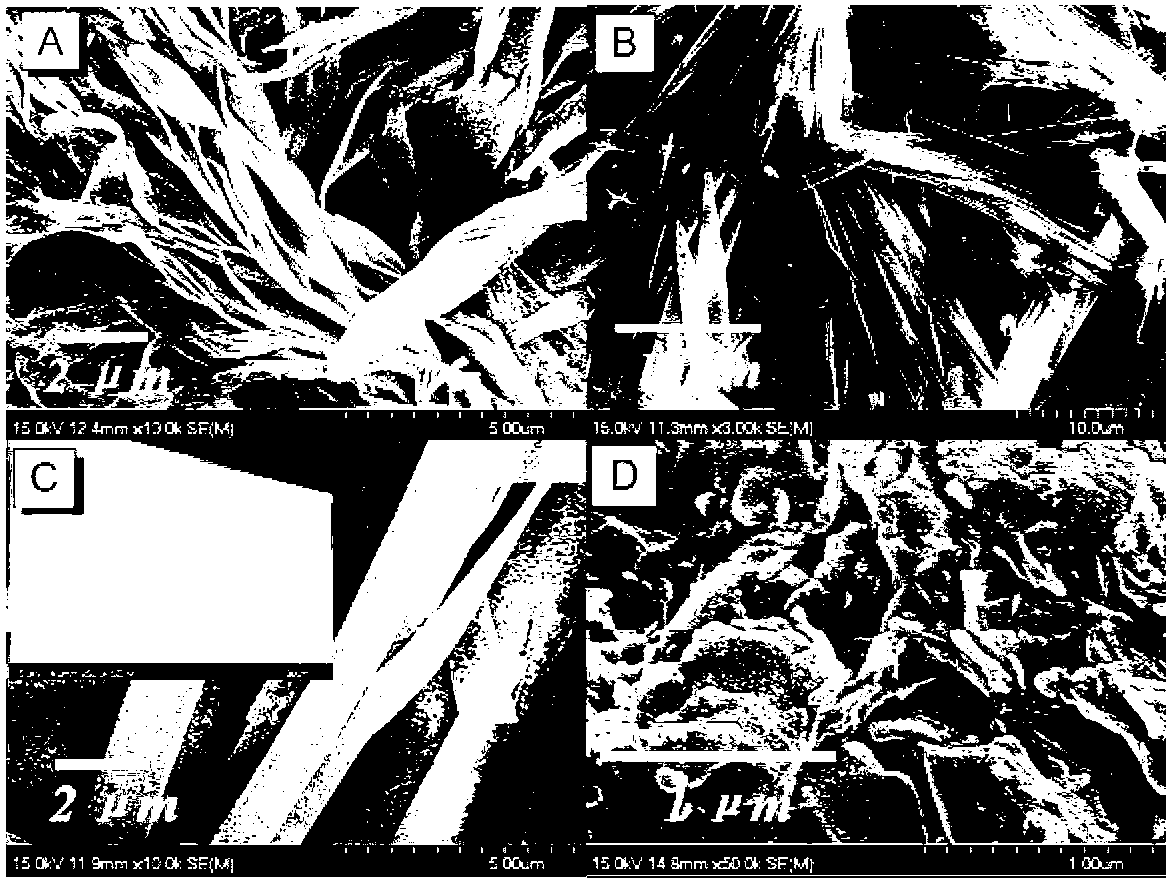
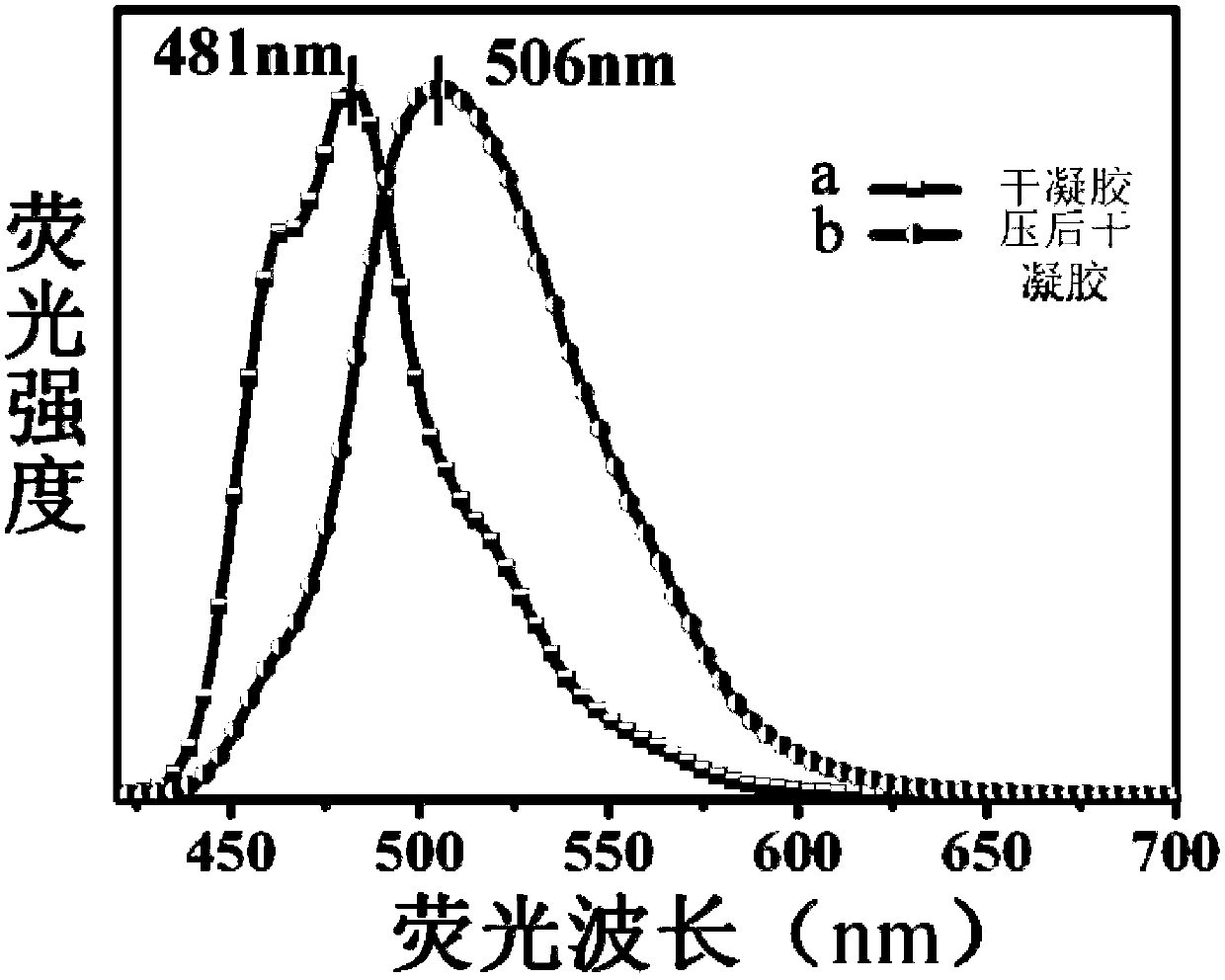
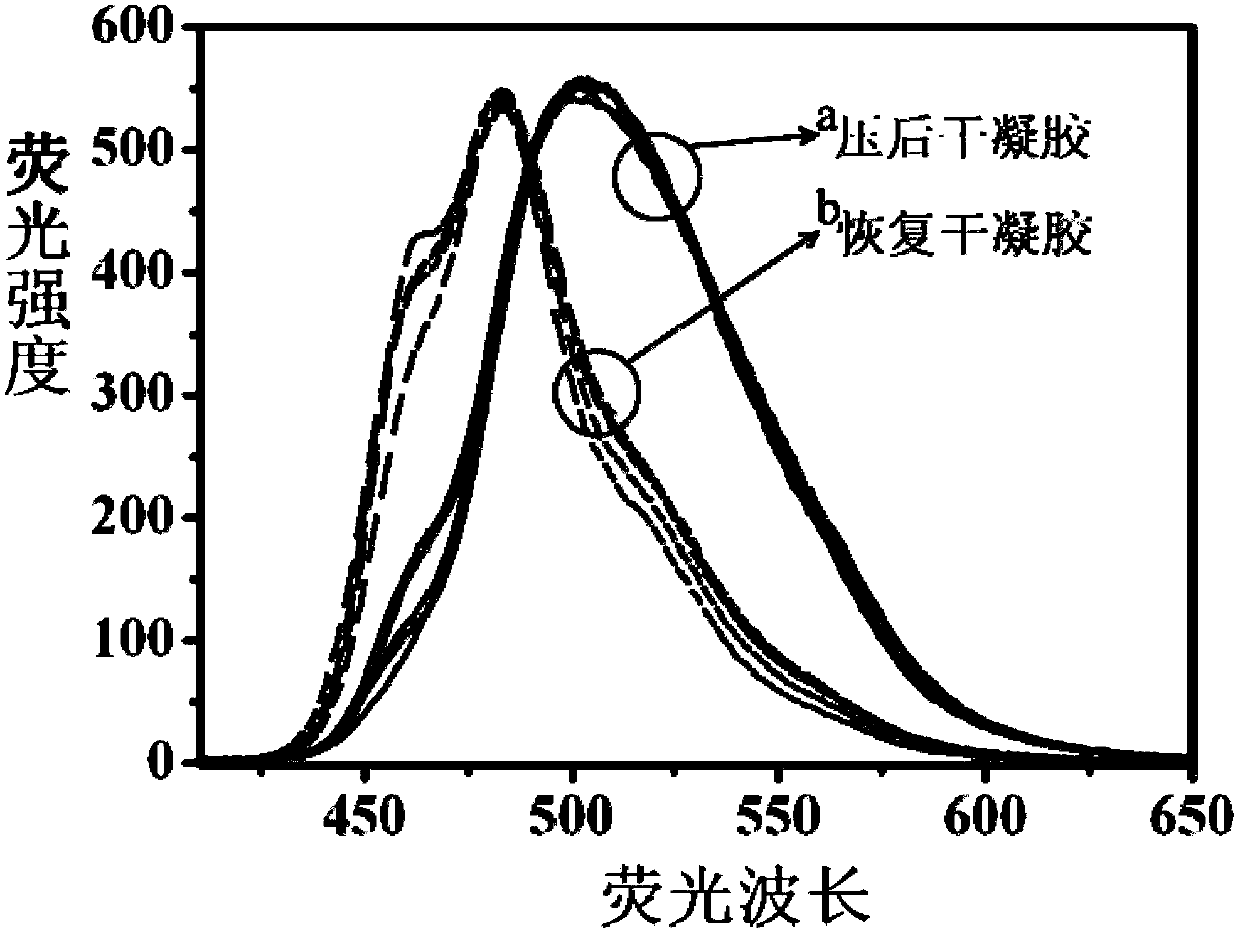
Triphenylamine derivatives as well as preparation method and application thereof
A derivative, triphenylamine technology, applied in the field of triphenylamine derivatives and its preparation and application, can solve problems such as limiting the application of materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of intermediate
[0037]Dissolve 3.47g (12mmol) of triphenylamine 4-boronate, 1.96g (10mmol) of p-bromonitrile benzyl, 0.11g (0.1mmol) of tetrakis(triphenylphosphine)palladium in a mixture of 50mL of toluene / 30mL of tetrahydrofuran, and add carbonic acid Aqueous sodium solution (2.0M, 3mL). Under the protection of nitrogen, raise the temperature to 90°C for 36 hours. After the reaction, cool the reaction solution to room temperature (25°C) and extract with chloroform. Take the lower layer of the extract (that is, the chloroform layer) and wash it with saturated brine. Dry with anhydrous magnesium sulfate, filter, and concentrate the filtrate to dryness under reduced pressure. The concentrate is subjected to silica gel column chromatography with a mixture of chloroform and petroleum ether at a volume ratio of 1:150, followed by TLC detection. The eluate of the product (III) was distilled to remove the solvent to obtain 2.76 g of a yellow po...
Embodiment 2
[0038] The preparation of embodiment 2 intermediate
[0039] Dissolve 3.47g (10mmol) of triphenylamine 4-boronate, 1.96g (10mmol) of benzyl p-bromonitrile, and 0.55g (0.5mmol) of tetrakis(triphenylphosphine)palladium in a mixture of 50mL toluene / 30mL absolute ethanol, Aqueous potassium carbonate solution (2.0 M, 5 mL) was added. Under the protection of argon, heat up to 90°C and react for 16h. After the reaction, cool the reaction solution to room temperature (25°C) and extract with chloroform. Take the extract from the lower layer, wash it with saturated saline, and dry it with anhydrous magnesium sulfate. , filtered, and the filtrate was concentrated to dryness under reduced pressure, and the concentrate was subjected to silica gel column chromatography using a mixture of chloroform and petroleum ether at a volume ratio of 1:150 as the eluent, followed by TLC detection, and the eluent containing the target product (III) was collected. The liquid was removed, and the solvent...
Embodiment 3
[0040] Preparation of triphenylamine derivatives shown in embodiment 3 formula (I)
[0041] Get 1.80g (5 mmol) of intermediate (III) prepared in Example 1, 2.07g (6mmol) of p-hexadecyloxybenzaldehyde and 0.03g (0.5mmol) of sodium methoxide and dissolve it in 30ml of chromatographically pure ethanol. Stir and react at (25°C) for 3 hours, a large amount of solid particles terminated the reaction, then put the reaction solution in a -20°C refrigerator overnight and then filter, the filter cake was rinsed with absolute ethanol three times and then dried to obtain a yellow-green powder product (ie (I) the triphenylamine derivative) 3.1g, the yield is 90%. The structural confirmation of the substance is characterized as follows: 1 H NMR (500 MHz, CDCl 3 ) δ(ppm) 7.89 (d, J=8.5 Hz, 2H), 7.70 (d, J=8.5 Hz, 2H), 7.63 (d, J=9.0 Hz, 2H), 7.50 (d, J=7.0 Hz, 2H), 7.49 (s, 1H), 7.28 (t, J=8.5 Hz, 4H), 7.14 (d, J=8.0 Hz, 6H), 7.05 (t, J=7.5 Hz, 2H), 6.97 (d, J=8.5 Hz, 2H), 4.02 (t, J=6.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com