Metformin hydrochloride gastric floating tablet and preparation method thereof
A technology of metformin hydrochloride and gastric floating tablets, which is applied in the field of biomedicine, can solve the problems of large doses and increased incidence of adverse reactions, and achieve long-lasting drug effects, reduced incidence of adverse reactions, and fast drug effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A metformin hydrochloride gastric floating tablet, which contains metformin hydrochloride, water-soluble resin, lactose, stearyl alcohol, sodium bicarbonate and magnesium stearate;
[0031] In this embodiment, each metformin hydrochloride gastric floating tablet contains 0.25 g of metformin hydrochloride, each tablet weighs 1.1375 g, and the parts by weight of each component according to the amount used for 1000 tablets are:
[0032]
[0033] The steps of preparing metformin hydrochloride gastric floating tablets by wet granulation:
[0034] A1: Material preparation: pass metformin hydrochloride through a 100-mesh sieve, and pass the rest of the pharmaceutical excipients through a 80-mesh sieve;
[0035] A2: Mixing materials: mix metformin hydrochloride, polymer gel, waxy material and foaming material in proportion;
[0036] A3: Granulation: Add 3-5% PVPK30 to the mixture obtained in step S2, and granulate through a 18-24 mesh sieve;
[0037] A4: Tablet compression...
Embodiment 2
[0043] A metformin hydrochloride gastric floating tablet contains metformin hydrochloride, water-soluble resin, lactose, stearyl alcohol, stearic acid, sodium bicarbonate and magnesium stearate.
[0044] In the present embodiment, each metformin hydrochloride gastric floating tablet contains 0.5 g of metformin hydrochloride, each tablet weighs 1.1 g, and the parts by weight of each component according to the amount used for 1000 tablets are:
[0045]
[0046]
[0047] Its preparation method is with embodiment 1. This embodiment is the best embodiment.
Embodiment 3
[0049] A metformin hydrochloride gastric floating tablet contains metformin hydrochloride, water-soluble resin, lactose, stearic acid, sodium bicarbonate and magnesium stearate.
[0050] In this embodiment, each metformin hydrochloride gastric floating tablet contains 0.75 g of metformin hydrochloride, with a total tablet weight of 1.7125 g, and the parts by weight of each component used in 1000 tablets are:
[0051]
[0052] Its preparation method is with embodiment 1.
[0053] Further illustrate effect of the present invention by experiment below:
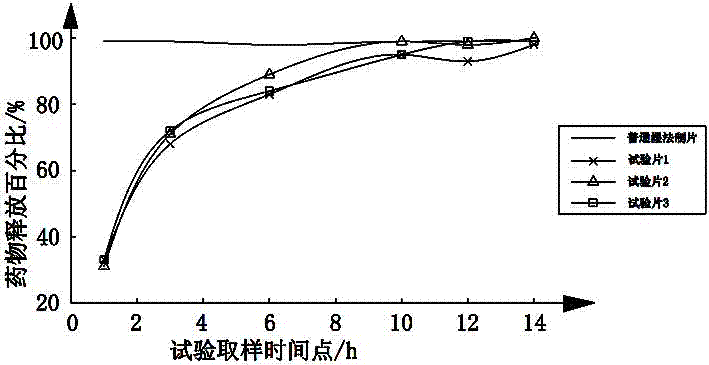
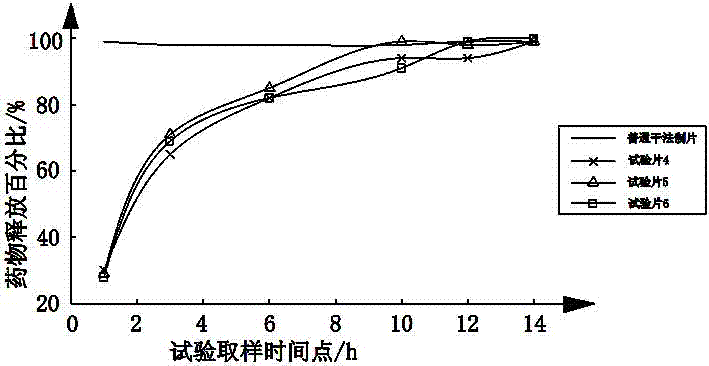
[0054] A kind of metformin hydrochloride gastric floating tablet that the embodiment of the present invention 1~3 makes is according to national drug standard WS 1 -(X-035)-2010Z measures the drug release rate, using 1000ml of phosphate buffer solution with pH 6.8 as the drug release medium, and the rotation speed is 100rpm, sampling and measuring the drug release rate at 1, 3, 6, 10, 12 and 14h percentage, as shown in the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com