Preparation method of nabumetone
A technology of nabumetone and acetone, which is applied in the field of preparation of nabumetone, can solve the problems of complicated process, long time, complicated preparation process, etc., and achieve the effect of simple process operation, few reaction steps, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
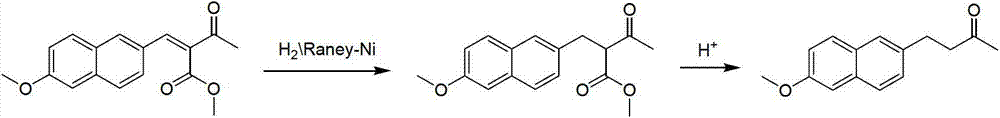
[0022] A preparation method for nabumetone, comprising the following steps:
[0023] (1) Dissolving 6-methoxyl-2-naphthaldehyde in acetone, using 10% sodium hydroxide aqueous solution as a catalyst, and reacting at a reaction temperature of 10 to 40° C. for 4 to 6 hours; then atmospheric distillation and concentration, Then distilled water is added to the concentrate to dilute, and the pH value is adjusted to neutrality with concentrated hydrochloric acid, and finally filtered to obtain the intermediate; wherein, the volume ratio of the 6-methoxyl-2-naphthaldehyde, acetone and sodium hydroxide is 6-methoxy-2-naphthaldehyde: acetone: sodium hydroxide = 6: 50: 1;
[0024] (2) Dissolve the intermediate obtained in (1) in ethyl acetate, use 5% of the mass of the intermediate as Raney nickel as a catalyst, pass through hydrogen, and heat the mixture at 20-30°C and 0.1MPa-4MPa Stir for 5h, then filter, concentrate the filtrate to half its volume, and finally recrystallize the remai...
Embodiment 1
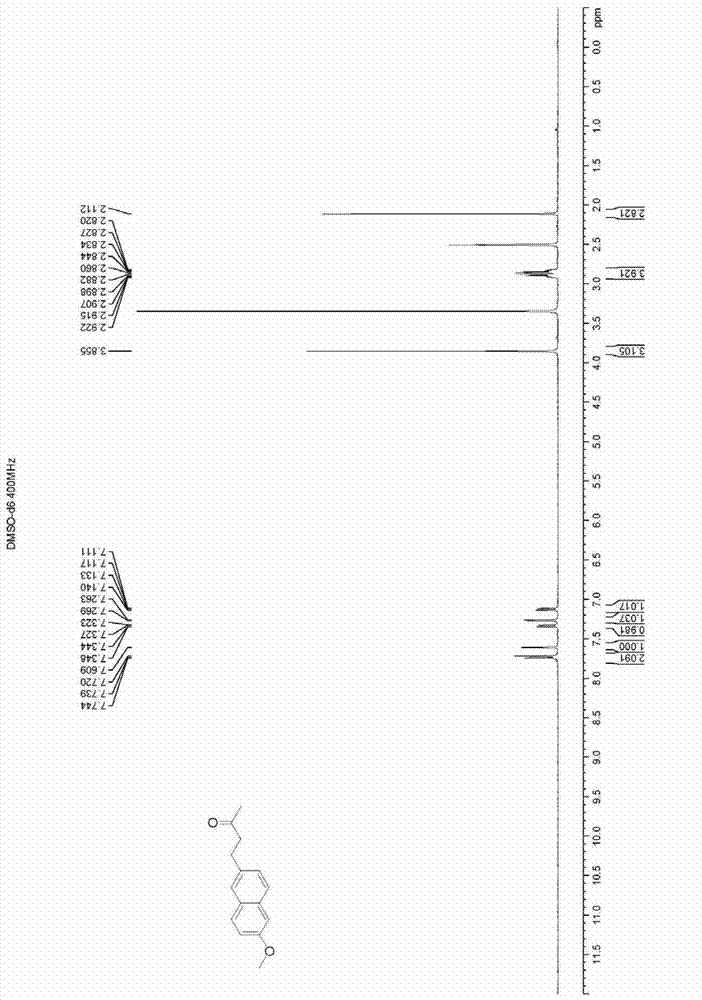
[0029] Dissolve 15 kilograms of 6-methoxy-2-naphthaldehyde in 250 liters of acetone, stir at a reaction temperature of 20°C, slowly drip 5 liters of 10% sodium hydroxide solution, and continue stirring for 4 hours; Collect two-thirds of the volume of acetone in the reaction solution, add about 100 liters of distilled water to the concentrate to dilute, and adjust the pH value to neutral with concentrated hydrochloric acid, that is, a large amount of yellow solids are precipitated, and 17.1 kg of intermediates are obtained by filtration. 4-(2-methoxynaphthalen-6-yl)but-3-en-2-one(4-(2-methoxynaphthalen-6-yl)-3-butene-2-one), the yield is 94 %;
[0030] Dissolve 17.1 kilograms of the intermediate in 120 liters of ethyl acetate, use 0.9 kilograms of Raney nickel as a catalyst, feed in hydrogen with a pressure of 2 MPa under stirring at 30°C, react for 5 hours, filter and recover the catalyst, and concentrate the filtrate to After half of the volume, it was cooled to zero, and a ...
Embodiment 2
[0032] 60 kilograms of 6-methoxyl-2-naphthaldehyde were dissolved in 1000 liters of acetone, stirred at a reaction temperature of 10°C, slowly dripped into 20 liters of 10% sodium hydroxide solution, and continued to stir for 5 hours; normal pressure distillation Collect 2 / 3 of the volume of acetone in the reaction solution, add about 400 liters of distilled water to the concentrate to dilute, and adjust the pH value to neutral with concentrated hydrochloric acid, that is, a large amount of yellow solids are precipitated, and 67.7 kg of intermediates are obtained by filtration. 4-(2-methoxynaphthalen-6-yl)but-3-en-2-one(4-(2-methoxynaphthalen-6-yl)-3-butene-2-one), the yield is 93 %;
[0033] Dissolve 67.7 kilograms of the intermediate in 480 liters of ethyl acetate, use 3.49 kilograms of Raney nickel as a catalyst, feed hydrogen with a pressure of 4 MPa under stirring at 20 ° C, react for 5 hours, filter and recover the catalyst, and concentrate the filtrate to After half of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com