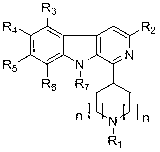
1,3,6,9-substitution beta-carboline compound and preparation method thereof
A compound and carboline technology, applied in the field of 1,3,6,9-substituted β-carboline compounds and their preparation, can solve the problems of fast growth of tumor cells, failure of chemotherapy, multidrug resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
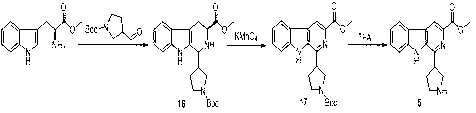
Examples
Embodiment 1
[0071] .
[0072] 1) Synthetic compound 9
[0073] In a 1L round bottom flask, tryptamine (32.0 g, 0.2 mol) was dissolved in 450 mL of acetonitrile, ethyl glyoxylate (43.5 g, 427.2 mmol) was added, the reaction was stirred at 80°C for 10 h, and the solution was spin-dried under vacuum After the crude product was obtained, the compound was obtained by silica gel column chromatography 9 (7.8 g, 16.3% yield).
[0074] 2) Synthetic compound 10
[0075] In a 250 mL round bottom flask, compound 9 (7.8 g, 32.5 mmol) and NaOH (2.6 g, 64 mmol) were dissolved in 40 mL ethanol and 80 mL water, the reaction was heated to reflux for 1 h, the ethanol was evaporated under reduced pressure, neutralized with concentrated hydrochloric acid until the pH value was 5, and cooled , filtered the precipitate to obtain the compound 10 (2.9 g, 42% yield). ESI-MS: 213.0 [M+H] + ; 1 H NMR (DMSO-d 6, 400 MHz ,): δ 11.89 (s, 1H), 8.54-8.50 (m, 2H), 8.39 (d, J = 8.0 Hz,1H), 7.89 (d...
Embodiment 2
[0079] .
[0080] 1) Synthetic compound 11
[0081] Add 5-methyl-indoleethylamine hydrochloride (300 g, 142.5 mmol) in 450 mL of acetonitrile in a 1L round bottom flask, add ethyl glyoxylate (43.5 g, 427.2 mmol) and ice to the solution Acetic acid (80 mL), the reaction was stirred at 40 °C for 10 h, the solution was spin-dried in vacuo, dissolved in DCM, and the residual acid was neutralized with saturated sodium bicarbonate solution, the organic layer was dried with anhydrous sodium sulfate, and the solvent was concentrated to dryness After obtaining the compound 11 It was directly used in the next reaction without purification.
[0082] 2) Synthetic compound 12
[0083] Compound in 500 mL round bottom flask 11 (34.5 g, 133.8 mmol) was dissolved in 240 mL of dichloroethane, sulfur powder (21 g, 656.1 mmol) was added, the reaction was stirred at 85°C for 10 h, the insoluble precipitate was removed by filtration, and a light yellow solid was obtained after...
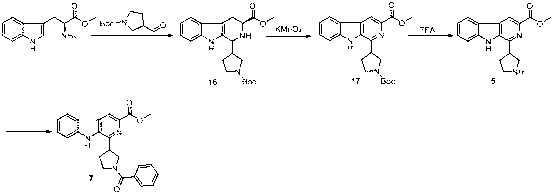
Embodiment 3
[0089] .
[0090] 1) Synthetic compound 14
[0091] Compound in 100 mL round bottom flask 12 (4.5 g, 17.7 mmol) was dissolved in 60 mL of tetrahydrofuran, 60% sodium hydrogen (2.1 g, 53.1 mmol) was added at 0°C, and after stirring for 1 h, methyl iodide (5 g, 35.4 mmol) was added, and the reaction solution was heated at room temperature After stirring overnight, neutralize with concentrated hydrochloric acid, obtain a yellow precipitate after filtration, and obtain a yellow powder compound after drying the precipitate 14 (3.9 g, 90.5% yield). ESI-MS: 240.8 [M+H] + , 1 H NMR (CD 3 OD, 400 MHz) d 8.52 (d, J = 8.0 Hz, 1H), 8.23 (d, J = 8.1 Hz, 1H), 8.14 (s, 1H), 7.66 (s, 2H), 4.21 (s, 3H), 2.56 (s, 3H).
[0092] 2) Synthetic compound 3
[0093] In a 10 mL reaction vial, compound 12 (70 mg) was dissolved in THF (2 mL), and 1.2 equivalents of benzylamine, 2 equivalents of triethylamine, 1.2 equivalents of EDCI and 1.5 equivalents of HOBT were ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com