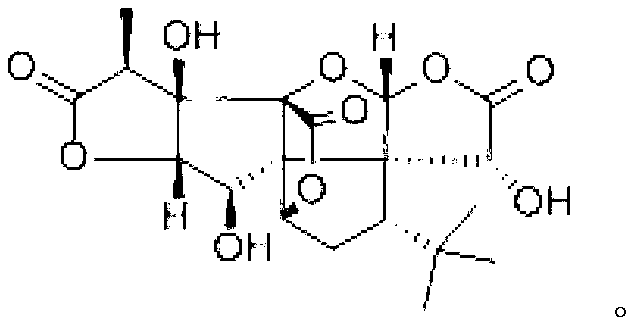
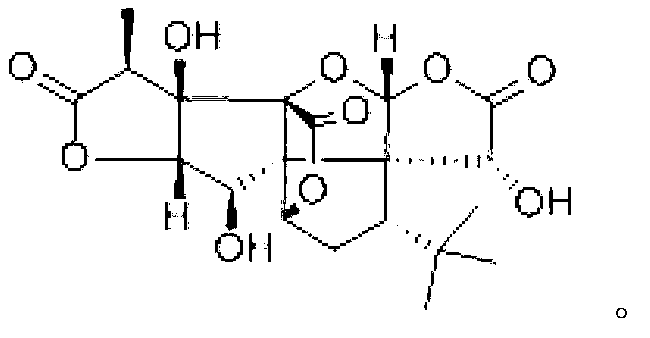
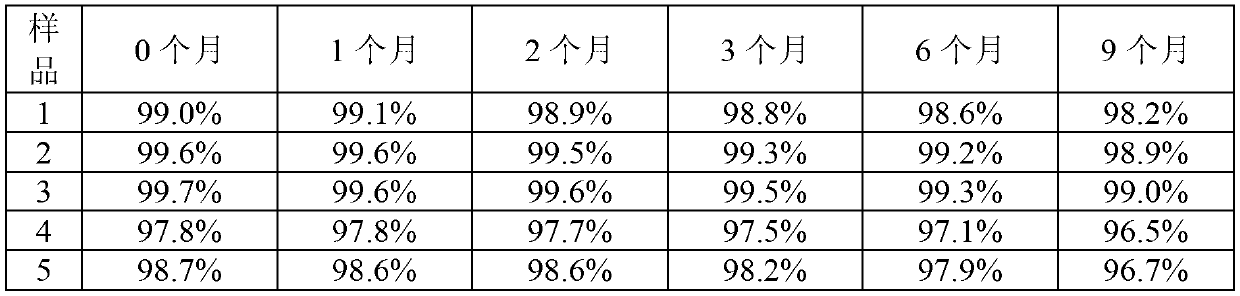
New bilobalide B compound and preparation method thereof
A technology of ginkgolide and compound, applied in the field of medicine, can solve the problems of insufficient ginkgolide B purity, poor ginkgolide B solubility, difficulty in ginkgolide B monomer, etc., and achieve good safety and controllable quality The effect of improving the bioavailability of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of ginkgolide B compound:
[0045] Add 40g of diatomaceous earth to 750ml of ethyl acetate under stirring conditions, then add 50g of Ginkgo biloba extract, heat and reflux at 85°C for 0.5 hours, filter, concentrate, and concentrate the filtrate to a liquid-solid ratio of 5ml: 1g; Add about 1 / 10 of the volume of distilled water to the concentrated solution to remove impurities, separate the ethyl acetate layer, and pass the ethyl acetate layer through an acidic alumina column. The diameter-to-height ratio of the acidic alumina column is 1:4, and the alumina is 200 mesh. The weight ratio of sample loading to alumina is 1:7, elute with saturated ethyl acetate at a rate of 1 / 4BV / h, and collect the eluate; the eluate is concentrated under reduced pressure until there is no ethyl acetate smell, Add an appropriate amount of ethanol to dissolve it, add water until the alcohol content is 60%, let it stand still, precipitate the crude crystals, filter, take the crude...
Embodiment 2
[0047] Preparation of ginkgolide B compound:
[0048] Add 40g of diatomaceous earth to 1000ml of ethyl acetate under stirring conditions, then add 50g of ginkgo biloba extract, heat and reflux at 78°C for 1.5 hours, filter, concentrate, and concentrate the filtrate to a liquid-solid ratio of 4ml:1g; Add about 1 / 9 volume of distilled water to the solution to remove impurities, separate the ethyl acetate layer, and pass the ethyl acetate layer through an acidic alumina column. The diameter-to-height ratio of the acidic alumina column is 1:4, and the alumina is 200 mesh. The weight ratio of sample volume to alumina is 1:7, elute with water-saturated ethyl acetate, the elution rate is 1 / 4BV / h, collect the eluate; the eluate is concentrated under reduced pressure until there is no ethyl acetate smell, add Appropriate amount of ethanol to dissolve it, add water until the alcohol content is 60%, let it stand still, precipitate the crude crystals, filter, take the crude crystals, and ...
Embodiment 3
[0050] Preparation of ginkgolide B compound:
[0051] Add 60g of diatomaceous earth to 850ml of ethyl acetate under stirring conditions, then add 50g of ginkgo biloba extract, heat and reflux at 80°C for 1 hour, filter, concentrate, and concentrate the filtrate until the liquid-solid ratio is 3ml:1g; Add about 1 / 11 volume of distilled water to the solution to remove impurities, separate the ethyl acetate layer, and pass the ethyl acetate layer through an acidic alumina column. The diameter-to-height ratio of the acidic alumina column is 1:10, and the alumina is 300 mesh. The weight ratio of the sample volume to alumina is 1:10, elute with saturated ethyl acetate with water, the elution rate is 1 / 2BV / h, collect the eluate; the eluate is concentrated under reduced pressure until there is no ethyl acetate smell, add Appropriate amount of ethanol to dissolve it, add water until the alcohol content is 70%, let it stand still, precipitate the crude crystals, filter, take the crude c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com