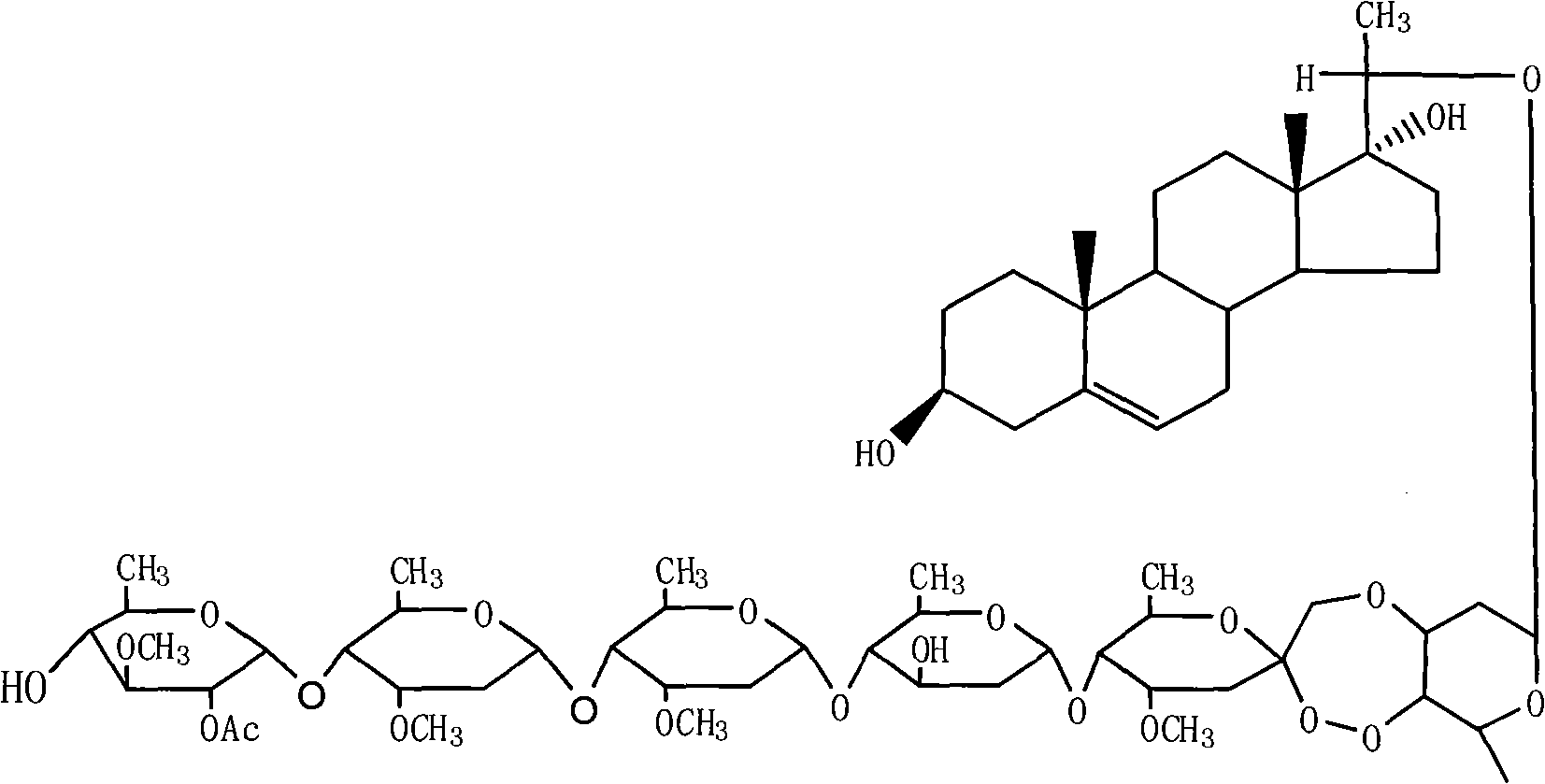
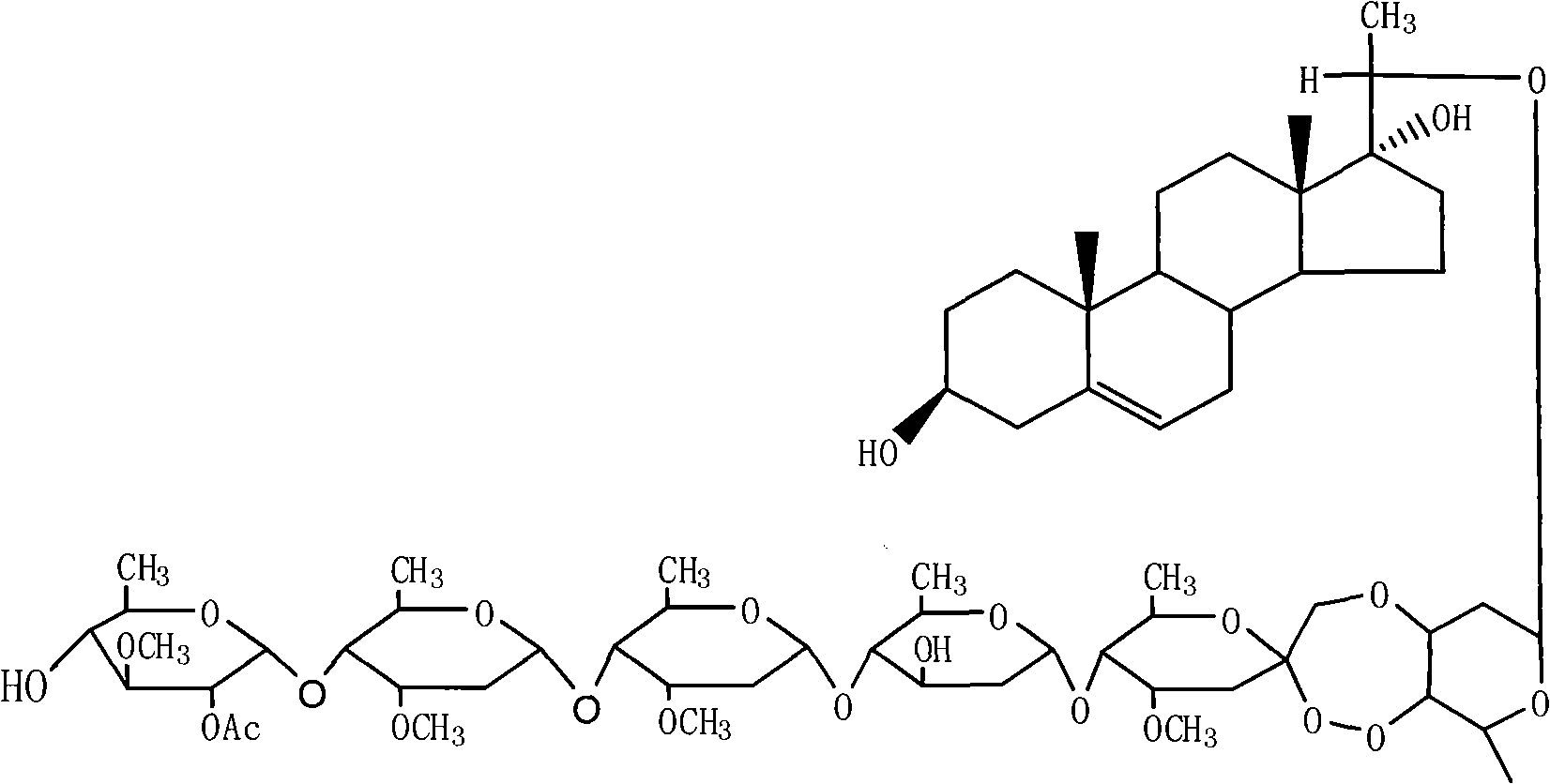
Periplocoside P-farm-oriented insecticidal active compound having activating effect on insect trypsin
A trypsin and activation technology, applied in the field of pesticides, can solve problems such as food safety threats and excessive pesticide residues, and achieve the effects of low pressure, large output, and good field control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] compound separation
[0011] Put 500g of the plant root bark and crushed plant sample in a 5L round bottom flask, add 3000mL methanol for reflux extraction for 3 hours, filter the supernatant, then add 3000mL methanol for reflux extraction for 2 hours, filter the supernatant liquid, the combined filtrates were concentrated under reduced pressure to about 100 mL. The concentrate was suspended in 400 mL of water, and then extracted three times with 500 mL of n-butanol, the butanol phases were combined, and concentrated to near dryness under reduced pressure. After the concentrate 60.0g chloroform was dissolved, the sample was mixed with silica gel, transferred to a silica gel column (4.0×100cm), and eluted with sherwood oil / acetone=9 / 1, 8 / 2, 7 / 3 (volume ratio) successively. Rinse 1000mL with each solvent ratio, collect one portion per 100mL, concentrate 6 / 4 of the eluent to dryness, transfer it to a silica gel column (3.0×50cm), and use petroleum ether / acetone=7 / 3, 6 / 4 (...
Embodiment 2
[0013] The effect of the compound barrella glucoside P on insect trypsin
[0014] The tryptase activity assay employs two obligate substrates: BaPNA and TAME. The strong alkaline trypsin activity was determined with BaPNA as the substrate, and the weak alkaline tryptase activity was determined with TAME as the substrate. BAPNA was dissolved in dimethyl alum at 10 mg / mL, and 40 μL was added to 0.5 ml 0.1 mol / L pH10.5 glycine-sodium hydroxide buffer solution containing midgut enzyme solution. After 20 minutes of reaction, 0.5 mL of 30% (Volume / Volume) Acetic acid was used to terminate the reaction, and the absorbance was measured at 405nm. TAME was dissolved in 0.15mol / L NaCl solution at 2mmol / L. Take 0.25ml of this solution and add 0.25ml of 0.1mol / L Tris-Hcl buffer solution with pH8.5 containing midgut enzyme solution, measure the light absorption change value at 247nm within 5min, and take a reading every 30min. The molar extinction coefficients of BaPNA and TAME are 8800M...
Embodiment 3
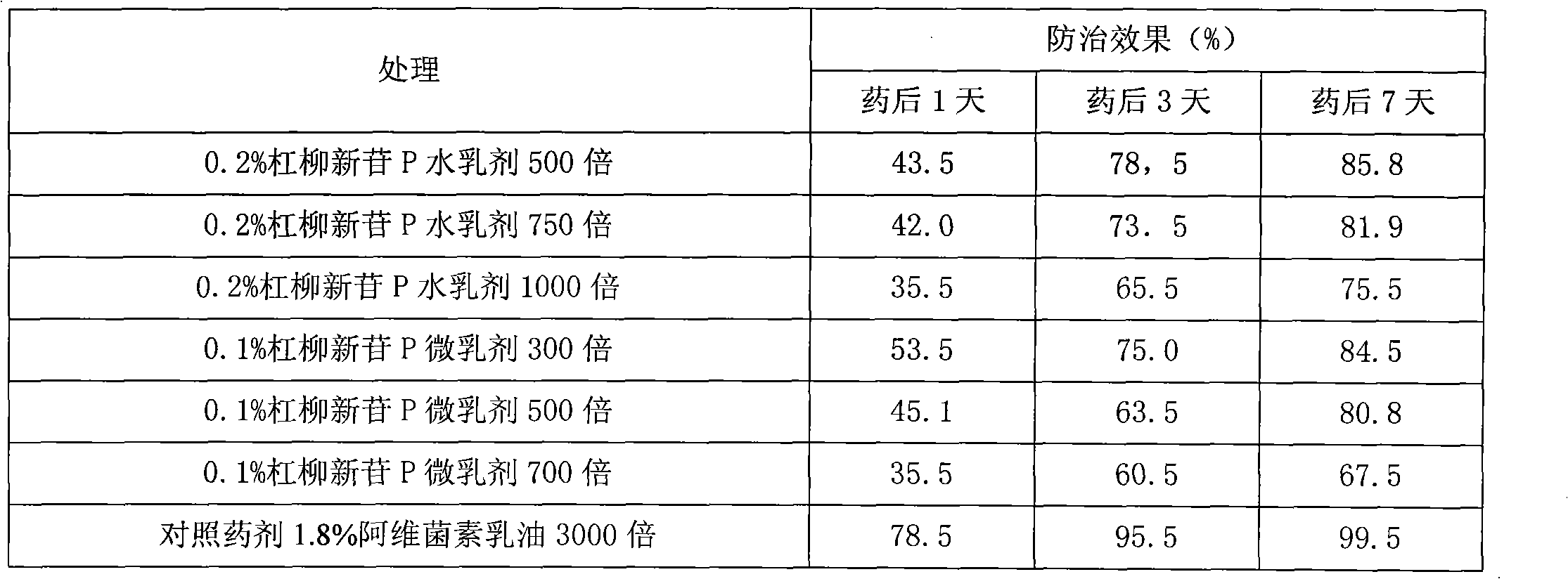
[0017] The Preparation of 0.2% Glycoside P Water Emulsion
[0018] Take by weighing the crude extract 16.0g containing the compound involved in the present invention (containing the compound ≥ 0.20g involved in the present invention) and dissolve it in 35g toluene, then add nonionic surfactant agricultural milk 4005.0g, TX-55.0g, water 35g, stirred until milky, namely the water emulsion with the compound as the active ingredient, wherein the active ingredient content is 0.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com