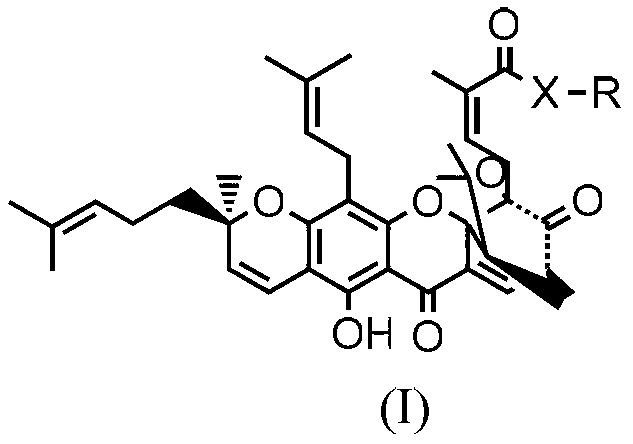
Gambogic acid ester derivative as well as preparation method and use thereof
A technology of gambogic acid and its derivatives, which is applied in the field of gambogic acid ester derivatives and their preparation, can solve the problems of adverse reactions and large immunosuppression, and achieve the effect of easy absorption, broad-spectrum anti-tumor activity, and stable chemical structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
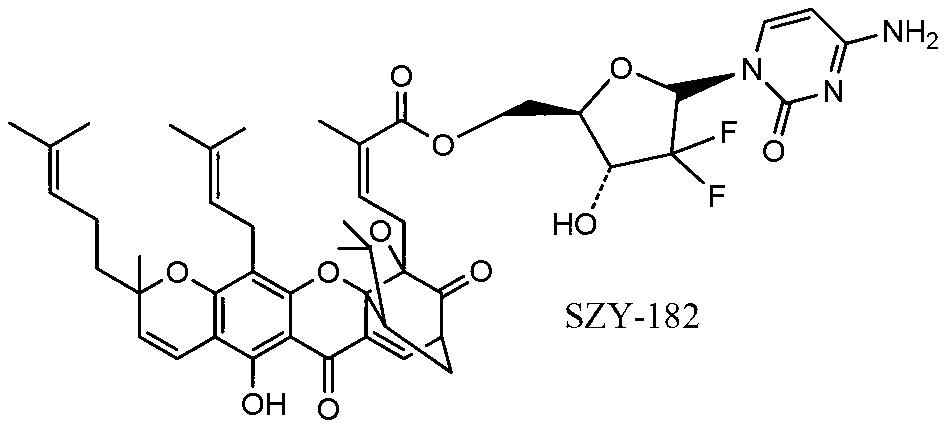
[0041] Synthesis of SZY-182
[0042] Under nitrogen protection, gambogic acid (500mg, 0.71mmol), gemcitabine (169mg, 0.64mmol) and triphenylphosphine (555mg, 2.13mmol) were dissolved in anhydrous tetrahydrofuran (5.0mL), cooled to 0°C for 10 minutes Inject a solution of DEAD (321mg, 2.13mmol) in 5ml THF with a syringe, return to room temperature naturally, and continue the reaction for 12h. Ethyl acetate (50ml) was added to the reactant to quench, then poured into saturated brine (3×10ml) for washing, dried over anhydrous sodium sulfate, and evaporated to remove ethyl acetate. Column chromatography [V (methanol): V (dichloromethane) = 3: 100] gave 294 mg of an orange-yellow amorphous powder with a yield of 47.4%. 1 H NMR (CDCl 3,400MHz)δ:12.87(s,1H,6-OH),7.58(d,J=6.88Hz,1H,10-H),6.68(d,J=10.12Hz,1H,4-H),6.38( m, 1H, 1”-H), 6.14~6.18(m, 1H, 27-H), 5.87(d, J=6.96, 2H, 5’-H, 6’-H), 5.40(d, J= 10Hz, 1H, 3-H), 5.06~5.08(m, 1H, 32-H, 37-H), 4.18(m, 1H, 4”-H), 4.08(m, 1H, 5”-H), ...
Embodiment 2
[0044] In vitro anti-tumor activity test
[0045] 1. Experimental cell line:
[0046] The tumor cell lines used in this experiment are: A549 (human lung adenocarcinoma cells), HCT116 (human colon cancer cells), PANC-1 (human pancreatic cancer cells) and HepG2 (human liver cancer cells) (provided by Shanghai Institute of Pharmaceutical Industry pharmacology laboratory cryopreservation and passage).
[0047] 2. Sample preparation:
[0048] After dissolving with DMSO (Merck), add PBS(-) to make a 1000μg / ml solution or a homogeneous suspension, and then dilute with DMSO-containing PBS(-). The positive control drug was gambogic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com