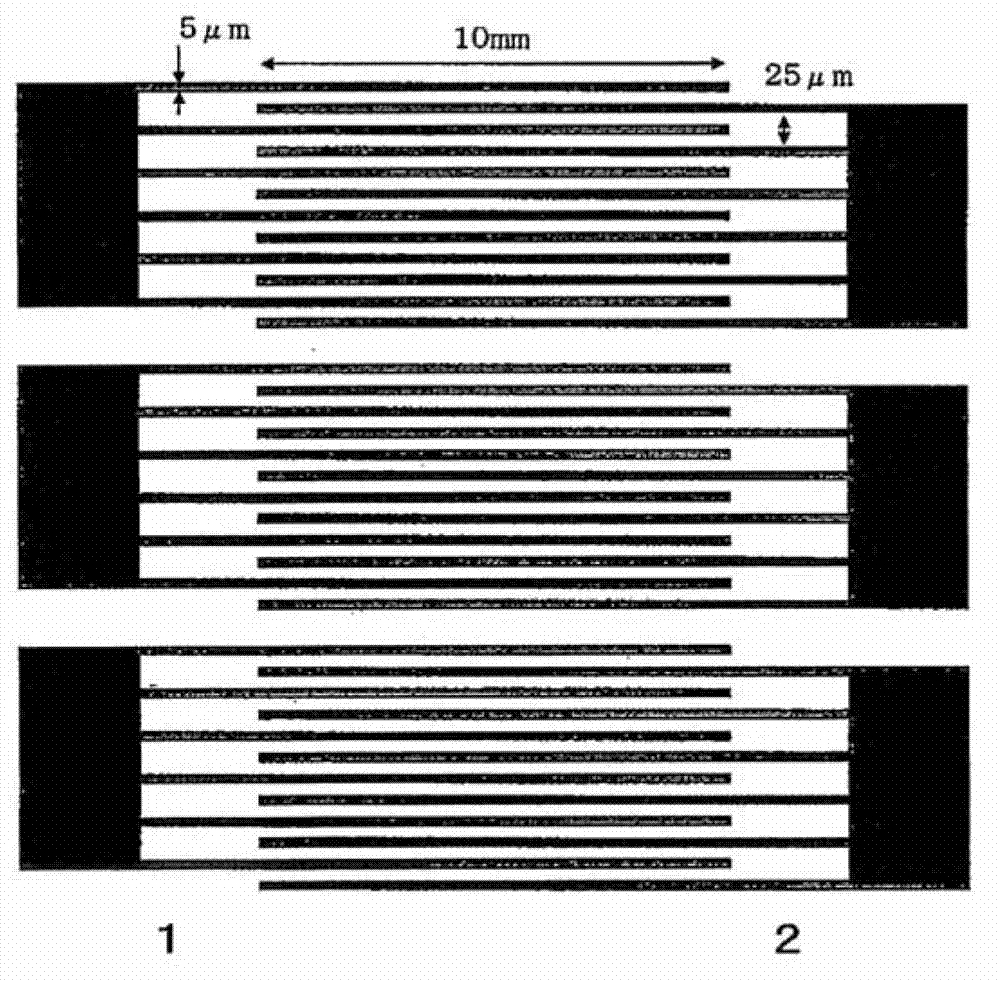
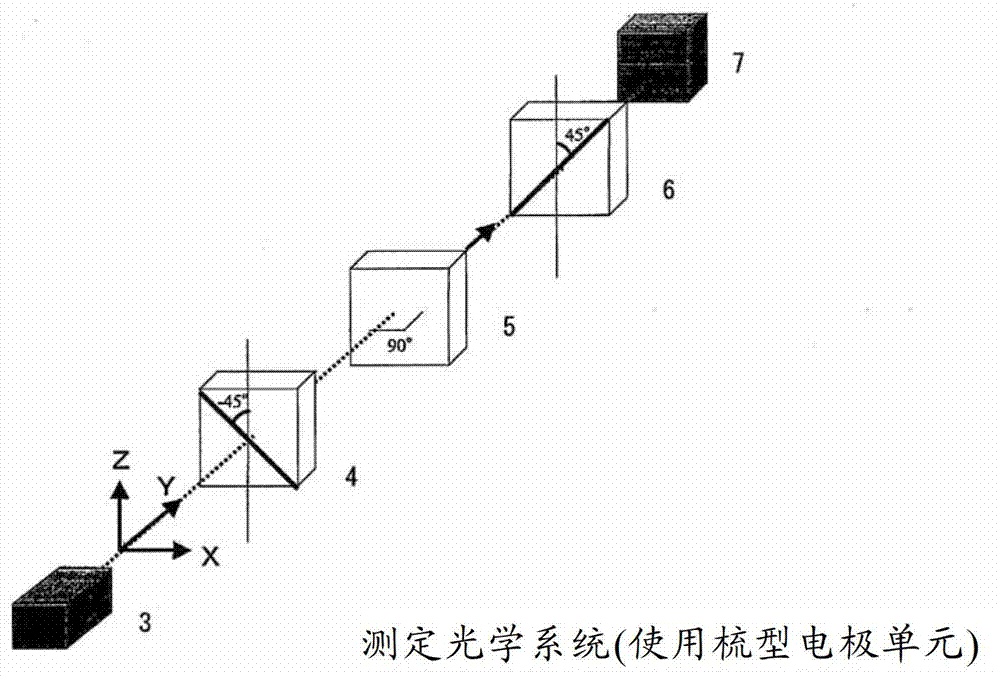
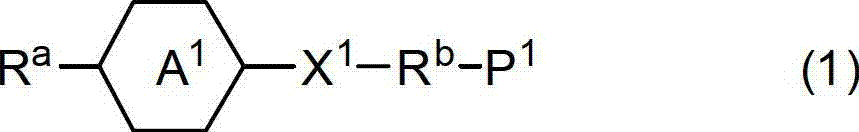Mixture of monomer and liquid crystal, polymer/liquid crystal composite material and liquid crystal element
A mixture and liquid crystal technology, applied in liquid crystal materials, optics, instruments, etc., can solve problems such as uneven concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0206] Preparation of compound (2-1)
[0207]
[0208] A base is used to etherify a phenol derivative and a halogen derivative, and an appropriate formyl chloride is allowed to act on the obtained compound to obtain the target compound.
[0209] In the formula, R a It has the meaning given by formula (2-1), and n is 1-30.
[0210] Preparation of compound (2-2)
[0211]
[0212] Hydrogen reduction is performed using a catalyst such as palladium in a compound obtained by acting on a Wittig reagent corresponding to a derivative of benzaldehyde. Next, an alcohol derivative is obtained by allowing a reducing agent such as LAH to act on the aldehyde derivative obtained by deprotecting the protecting group. Finally, the desired compound can be obtained by reacting the alcohol derivative with an appropriate formyl chloride.
[0213] In the formula, R a It has the meaning given by formula (2-2), and n is 1-30.
[0214] Preparation of compound (2-3)
[0215]
[0216] ...
example
[0281] Hereinafter, the present invention will be described more specifically by way of examples, but the present invention is not limited by these examples.
[0282] In the examples of this specification, I represents the non-liquid crystal isotropic phase, N represents the nematic phase, and N * Indicates a chiral nematic phase, BP indicates a blue phase, and BPX indicates an optically isotropic liquid crystal phase in which diffracted light of two or more colors is not observed. In this specification, the I-N phase transition point is sometimes referred to as the N-I point. sometimes I-N * The phase transition point is called N * -I point. The I-BP phase transition point is sometimes referred to as the BP-I point.
[0283] Synthesis examples of monofunctional monomers used in the present invention are shown below.
example 1
[0284] Example 1: Synthesis of 8-(4-pentylphenoxy)-octyl acrylate
[0285]
[0286] 4-amylphenol (24.3g, 148.2mmol) was dissolved in dimethylformamide (Dimethylformamide, DMF) (100ml), then 8-chlorooctyl alcohol (24.4g, 148.2mmol), potassium carbonate (41.0g , 296.4mmol), and heated and stirred at 80° C. for 30 hours under a nitrogen atmosphere. After completion of the reaction, water was added to further make the solution acidic, and then toluene was added for extraction. After washing with water and a saturated aqueous sodium chloride solution, it was dried over anhydrous magnesium sulfate, and the solvent was concentrated under reduced pressure. Purification was carried out by silica gel column chromatography to obtain compound (S-01) (28.3 g, 65%).
[0287] Compound (S-01) (15.0 g, 51.3 mmol) was dissolved in tetrahydrofuran (THF) (130 ml), and then triethylamine (8.6 ml, 61.6 mmol) was added. After cooling to 0°C, acryloyl chloride (5.0ml, 61.6mmol) was added dropwi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Interval | aaaaa | aaaaa |
| Phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com