Drug carrier system and preparation method thereof
A carrier system and drug technology, applied in the field of drug carrier system and its preparation, can solve the problems of normal tissue and organ side effects, difficult treatment, and reduced drug availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] The invention provides a preparation method of a drug carrier system, comprising the following steps:
[0057] A) Mix and incubate the negatively charged drug with the cationic carrier to obtain a binary complex;
[0058] B) mixing the binary complex with a pH-sensitive masking system to obtain a drug carrier system;
[0059] The pH sensitive masking system is a copolymer of hyperbranched polyethyleneimine, lysine and glutamic acid, or a copolymer of hyperbranched polyethyleneimine, lysine and aspartic acid; The molecular weight of the copolymer of polyethyleneimine, lysine and glutamic acid is 2600~60000; The molecular weight of the copolymer of described hyperbranched polyethyleneimine, lysine and aspartic acid is 2600~ 60000; in the hyperbranched polyethyleneimine, lysine and glutamic acid copolymer, the molar ratio of glutamic acid to lysine is (1.5-50): 1; the hyperbranched In the copolymer of polyethyleneimine, lysine and aspartic acid, the molar ratio of aspart...
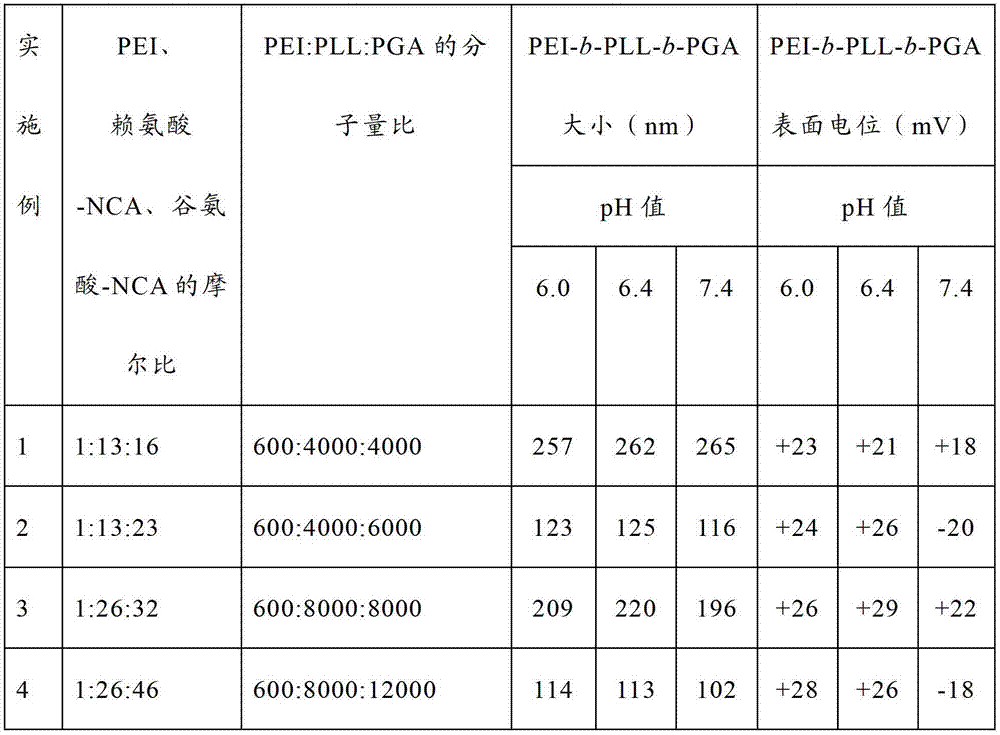
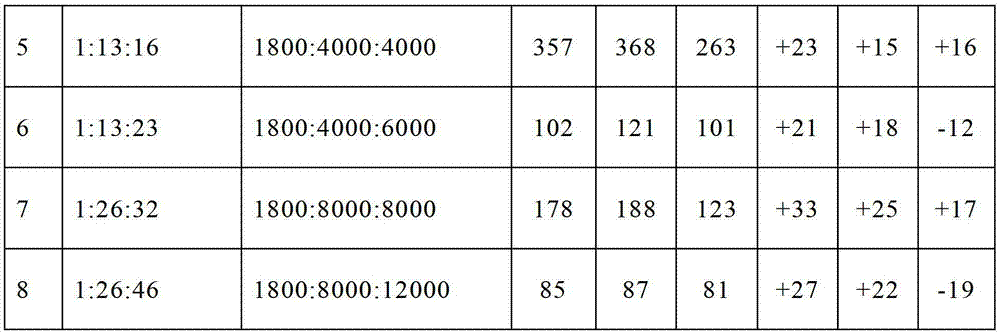
Embodiment 1~8
[0068] Dissolve polyethyleneimine (PEI) and lysine-N-endocarboxylic acid anhydride in a mixed solvent of dichloromethane and DMF, respectively, and the volume ratio of dichloromethane and DMF is 1:2. Both were mixed and reacted at 30° C. for 72 hours. Then add the mixed solution of ε-benzyloxycarbonyl-L-glutamic acid-N-internal carboxylic acid anhydride in dichloromethane and DMF, react at 35°C for 72 hours, settle with ether after the reaction, filter and dry, dissolve in trifluoro Add the acetic acid solution of hydrogen bromide to acetic acid, deprotect at room temperature for 2 hours, then settle with ether, dissolve in water after drying, dialyze with a 3500Da dialysis bag for 3 days, change the water 6 times, and obtain polyethylene glycol after freeze-drying. The block copolymer of amine-polylysine-polyglutamic acid is denoted as PEI-b-PLL-b-PGA.
[0069] PEI-b-PLL-b-PGA with different molecular weights and compositions can be obtained by changing the molar amounts of ...
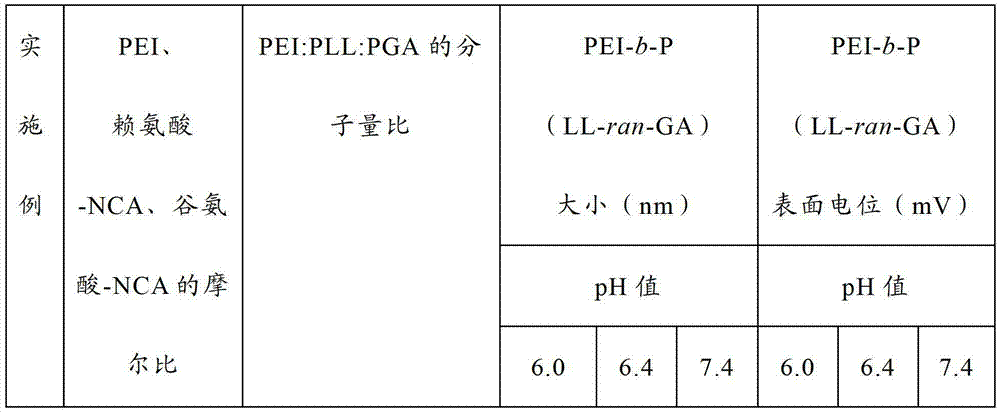
Embodiment 9~16
[0074] According to the raw material ratio shown in Table 1, the random copolymer of polyethyleneimine-polylysine-polyglutamic acid was prepared according to the following method.
[0075] Dissolve polyethyleneimine (PEI) in a mixed solvent of dichloromethane and DMF, the volume ratio of dichloromethane and DMF is 1:2, add lysine-N-endocarboxylic acid anhydride and ε-benzyloxycarbonyl- L-glutamic acid-N-internal carboxylic acid anhydride, react at 35°C for 72 hours, settle with ether after the reaction, filter and dry, dissolve in trifluoroacetic acid, add hydrogen bromide in acetic acid solution, deprotect at room temperature 2 hours, then settled with ether, dried and dissolved in water, dialyzed with a 3500Da dialysis bag for 3 days, changed the water 6 times, and obtained a random copolymer of polyethyleneimine-polylysine-polyglutamic acid after freeze-drying the product , recorded as PEI-b-P (LL-ran-GA).
[0076] PEI-b-P (LL-ran-GA) with different molecular weights and c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com