Preparation method of 7beta-amino-7lapha-methoxy-3-cephem compound
A compound, methoxy technology, applied in the field of preparation of pharmaceutical raw materials 7β-amino-7α-methoxy-3-cephem compound, can solve the problems of long steps, many steps, low yield, etc., and achieve easy reaction , fewer side reactions, good reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
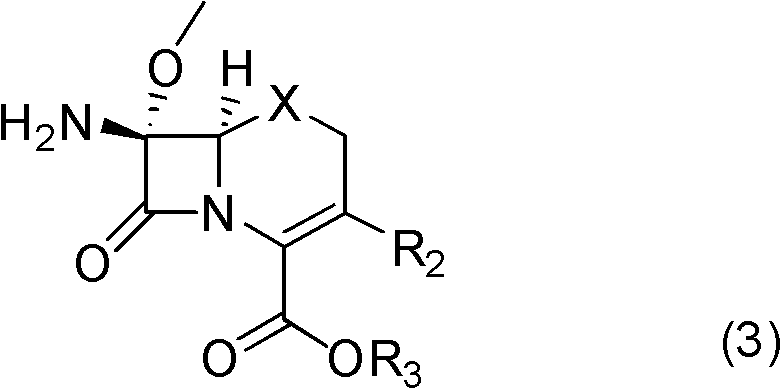
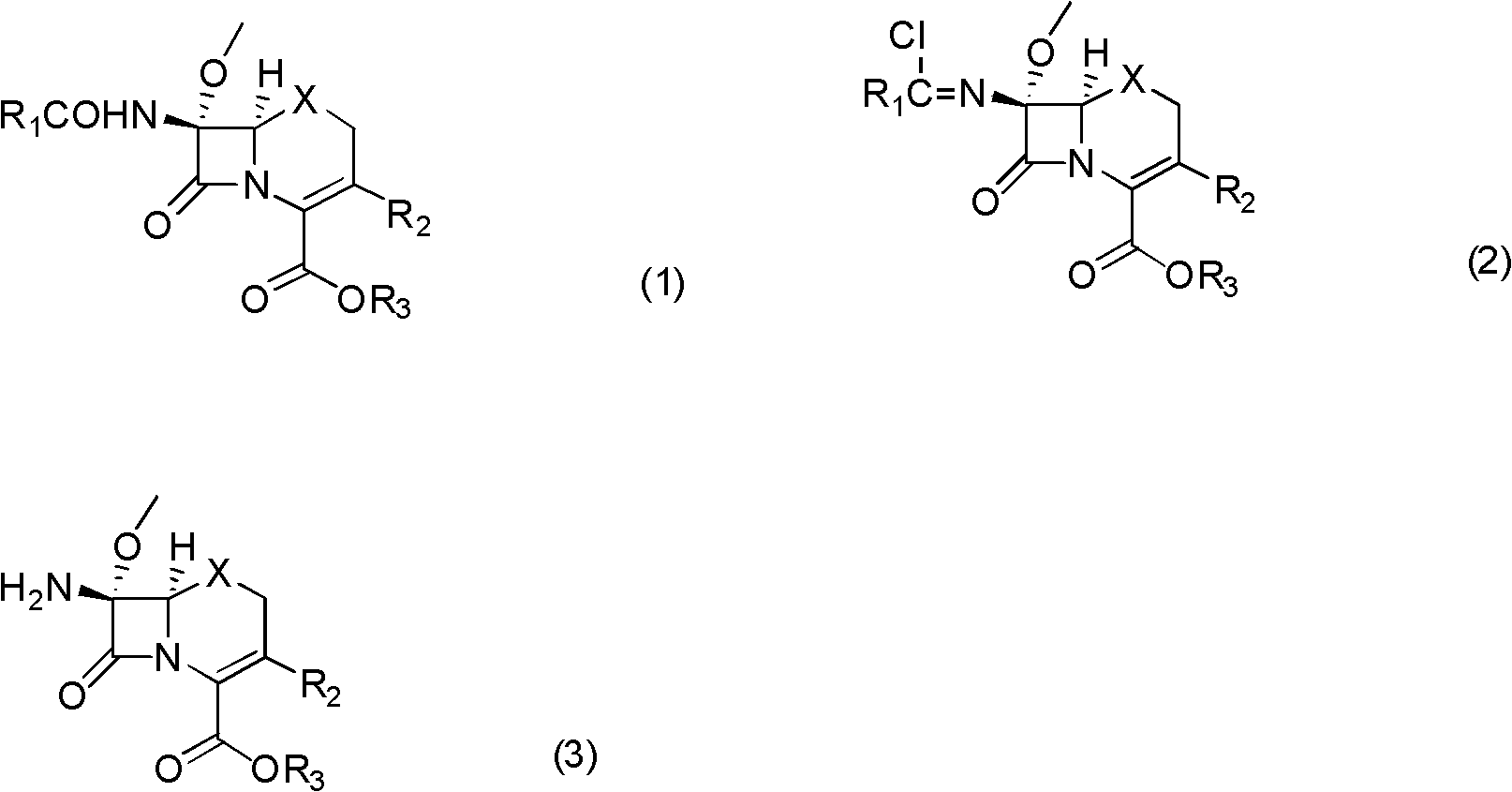
[0043] Example 1: 7β-amino-7α-methoxy-3(1-methyl-1H-tetrazol-5-ylthiomethyl)-1-oxo-3-cephem-4-carboxylic acid di Preparation of Benzyl Ester
[0044] In a 1000mL three-neck flask with mechanical stirring and a thermometer, add 500mL of dichloromethane, and drop 30.7g (0.05mol) of compound (1a) (R 1 = phenyl, R 2 = 1-methyl-1H-tetrazol-5-ylsulfomethylene R 3 =-CH(C 6 h 5 ) 2 , X=O), add 15.2g (0.15mol) of triethylamine, stir to dissolve, protect with nitrogen, cool to -30°C with a cryogenic bath, add 14.9g (0.05mol) of bis(trichloromethyl)carbonate in batches , control the temperature not higher than -15°C, add in 0.5 to 1 hour, slowly raise the temperature to 0°C for 1 hour and react for 2 hours; after the reaction is completed, cool to -30°C in a low-temperature bath, add methanol 32g (1mol) dropwise in 15 minutes ), continue to react at -20~-10°C for 1 hour; warm up to -5°C, add saturated sodium bicarbonate solution dropwise to pH=7-8; separate the organic layer, and...
Embodiment 2
[0045] Example 2: 7β-amino-7α-methoxy-3(1-methyl-1H-tetrazol-5-ylthiomethyl)-1-oxo-3-cephem-4-carboxylic acid di Preparation of Benzyl Ester
[0046] In a 1000mL three-neck flask with mechanical stirring and a thermometer, add 400mL of dichloromethane, and drop 30.7g (0.05mol) of compound (1a) (R 1 = phenyl, R 2 = 1-methyl-1H-tetrazol-5-ylsulfomethylene R 3 =-CH(C 6 h 5 ) 2, X=O), add pyridine 15.8g (0.2mol), stir to dissolve, nitrogen protection, cool to -25°C with a cryogenic bath, add bis(trichloromethyl)carbonate 19.8g (0.0667mol) in batches, control The temperature is not higher than -10°C, the addition is completed in 0.5 to 1 hour, and the temperature is slowly raised to 5°C for 1 hour to react for 2 hours; after the reaction is completed, cool to -25°C in a low-temperature bath, and add methanol 32g (1mol) dropwise in 15 minutes. Continue to react at -20~-10°C for 1 hour; warm up to -5°C, add saturated sodium bicarbonate solution dropwise to pH = 7-8; separate ...
Embodiment 3
[0047] Example 3: 7β-amino-7α-methoxy-3(1-methyl-1H-tetrazol-5-ylthiomethyl)-1-oxo-3-cephem-4-carboxylic acid di Preparation of Benzyl Ester
[0048] In a 1000mL three-neck flask with mechanical stirring and a thermometer, add 400mL of dichloroethane, and drop 30.7g (0.05mol) of compound (1a) (R 1 = phenyl, R 2 = 1-Methyl-1H-tetrazol-5-ylsulfomethylene R 3 =-CH(C 6 h 5 ) 2 , X=O), add 11.4 g (0.1 mol) of N, N'-dimethylpiperazine, stir to dissolve, protect with nitrogen, cool to -25°C with a cryogenic bath, add bis(trichloromethyl)carbonic acid in batches Ester 19.8g (0.0667mol), control the temperature not higher than -10°C, add in 0.5-1 hour, slowly raise the temperature to 5°C for 2 hours and react for 2 hours; after the reaction, cool to -25°C in a low-temperature bath for 15 minutes Add methanol 32g (1mol) dropwise, continue to react at -10~0°C for 1 hour; warm up to 5°C, add saturated sodium bicarbonate solution dropwise until the pH value = 7-8; separate the orga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com