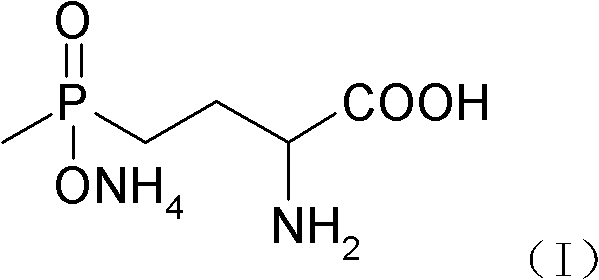
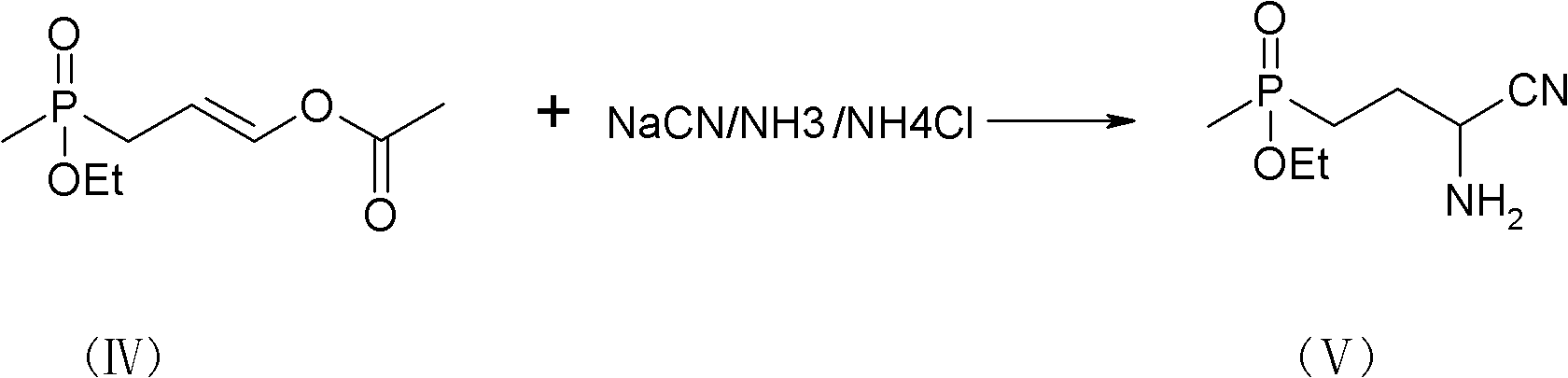Glufosinate-ammonium preparation method
A technology of glufosinate and diethyl methylphosphonite, applied in the field of improved preparation of glufosinate and its derivatives, can solve the problem of low actual yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: compound (VIII) preparation
[0036] (1) Synthesis of compound (IV)
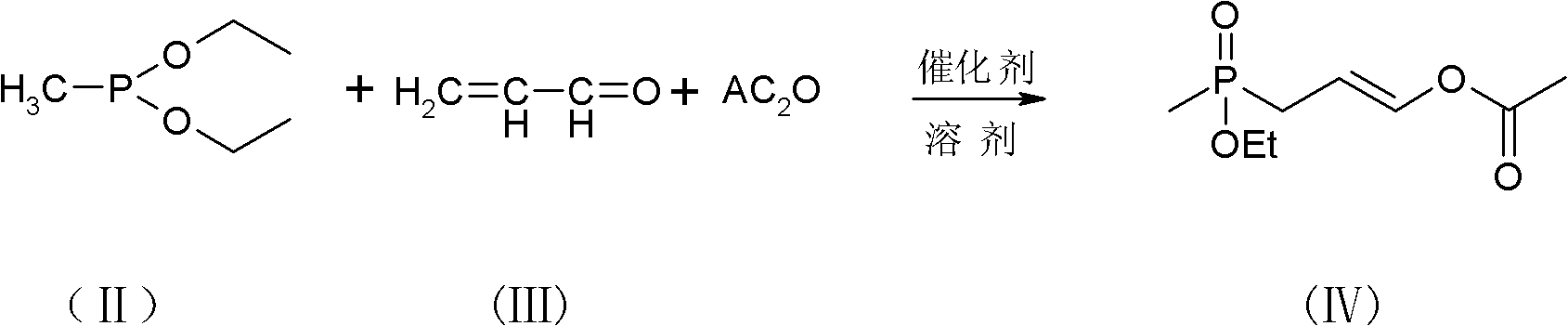
[0037] Add 0.1 mole of diethyl methylphosphonite, 30 ml of tetrahydrofuran, and 0.2 g of hexamethylphosphoric triamide (referred to as "HMPA") to the dry three-neck flask after anaerobic treatment at 20-25 °C, and stir Continuously drop the mixture of 0.1 mole of acrolein and 0.1 mole of acetic anhydride. During the dropping process, keep the internal temperature below 25°C. The reaction is an exothermic process. Control the dropping time within 1-3 hours. Insulate and react at 25-30° C. for 2-4 hours, and analyze by GC whether the raw material diethyl methylphosphonite has reacted completely to obtain the product (IV).
[0038] (2) Synthesis of compound (V)
[0039] Add the addition product (IV) dropwise to the solution of sodium cyanide and ammonium chloride at a temperature of 10-30°C. After the dropwise addition is completed, keep the temperature at 30°C for 1 hour. n-Butanol-Tolu...
Embodiment 2
[0046] Embodiment 2: compound (VIII) preparation
[0047] In an anaerobically treated dry three-neck flask, add 0.5 g of N,N-dimethylformamide to a mixture of 0.1 mole of diethyl methylphosphinate, 0.1 mole of ethanol and 0.1 mole of tetrahydrofuran, and at room temperature Add freshly distilled 0.1 mole of acrolein to 0.1 mole of acetic anhydride, and add dropwise to the diethyl methylphosphonite solution at 25-30°C. The mixture was stirred at 30°C for 2 hours, then added dropwise to an aqueous solution of 0.1 mol sodium cyanide and 0.2 mol ammonium chloride at 25-28°C, and after keeping warm for 1 hour, 50 ml of 28% ammonia water was added. React at 30°C for 2 hours, add the thickened aminonitrile to 200 ml of 37% hydrochloric acid, heat the mixture under reflux for 2 hours, and distill off ethanol and acetic acid. The mixture was concentrated with a rotary evaporator, and the pH value was adjusted to about 9 with ammonia water. The product content was analyzed by liquid ch...
Embodiment 3
[0048] Embodiment 3: compound (VIII) preparation
[0049]In an anaerobically treated dry there-necked flask, 0.2 g of hexamethylphosphoric triamide was added to the mixture of 0.1 mole of diethyl methylphosphinate, 0.1 mole of ethanol and 0.1 mole of ethylene glycol dimethyl ether, Add freshly distilled 0.1 mol acrolein to 0.1 mol acetic anhydride at room temperature, and add dropwise to the diethyl methylphosphonite solution at 25-30°C. The mixture was stirred at 30°C for 2 hours, then added dropwise to an aqueous solution of 0.1 mol sodium cyanide and 0.2 mol ammonium chloride at 25-28°C, and after keeping warm for 1 hour, 50 ml of 28% ammonia water was added. React at 30°C for 2 hours, add the thickened aminonitrile to 200 ml of 37% hydrochloric acid, heat the mixture under reflux for 2 hours, and distill off ethanol and acetic acid. The mixture was concentrated with a rotary evaporator, and the pH value was adjusted to about 9 with ammonia water. The content of the produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com