Synthetic method of acetaldehyde dehydrogenase activator alda-1, and application of same
A technology of acetaldehyde dehydrogenase and activator, which is applied in the field of biomedicine, can solve the problems that the new compound alda-1 has few reports, no research, and cannot effectively enter the brain and central nervous system, so as to simplify the synthesis route, The effect of expanding indications and enhancing compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Synthesis of alda-1
[0026] Add 8.50g (56.2mmol) of piperonylamine into a 500ml round bottom flask equipped with magnetic stirring, add 100ml of N,N'-dimethylformamide (DMF), cool and stir in an ice-water bath for 10 minutes, add 2,6- Dichlorobenzoic acid 11.77g (61.6mmol), dicyclohexylcarbodiimide (DCC) 12.95g (62.8mmol), 1-hydroxybenzotriazole (HOBT) 1.52g (11.3mmol), continue stirring for 30 minutes, remove Ice-water bath, stirring at room temperature for 12 hours. Thin-layer chromatography detects that the conversion of 2,6-dichlorobenzoic acid is complete, cooling and stirring in an ice-water bath for 1 hour, filtering, and washing the filter cake with 20ml of ice-cold DMF, combining the filtrate and washings, and transferring to a 1000ml three-necked round-bottomed flask with mechanical stirring 600ml of deionized water was added dropwise within 2 hours. After the dropwise addition was completed, the stirring was continued for 1 hour, filtered, and th...
Embodiment 2ald
[0028] Embodiment 2 preparation of alda-1 compressed tablet
[0029] Table 1 prepares the used raw materials of alda-1 compressed tablet and the dosage used for pressing 1000 tablets
[0030]
[0031]
[0032] Preparation process: mix alda-1, microcrystalline cellulose, crospovidone and magnesium stearate evenly, and then compress into tablets.
Embodiment 3
[0033] Embodiment 3: animal experiments
[0034] The distribution of whole body tissues at different time after gavage of alda-1 in rats
[0035] Table 2 Changes of drug content in various tissues at different times after gavage of alda-1 to rats
[0036] (ng equivalent / g or mL, mean ± SD, n=5)
[0037]
[0038] The distribution of total radioactivity at different times after gavage of alda-1 to rats is shown in Table 2. The distribution of total radioactivity in plasma and several important tissues at different times after intragastric administration of alda-1 was as follows: plasma, kidney, heart, liver, and brain. Less spleen, muscle, fat and bone marrow.
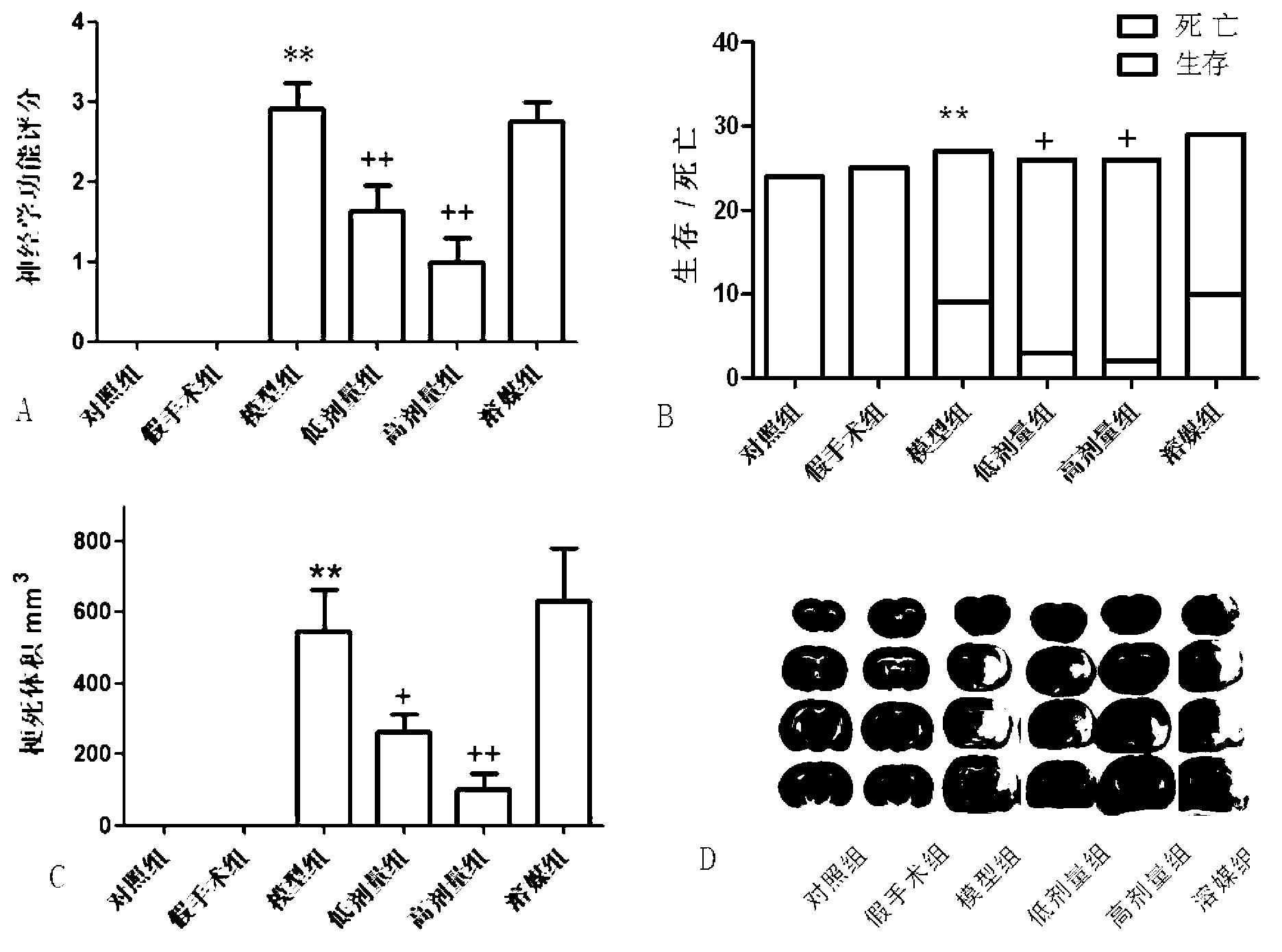
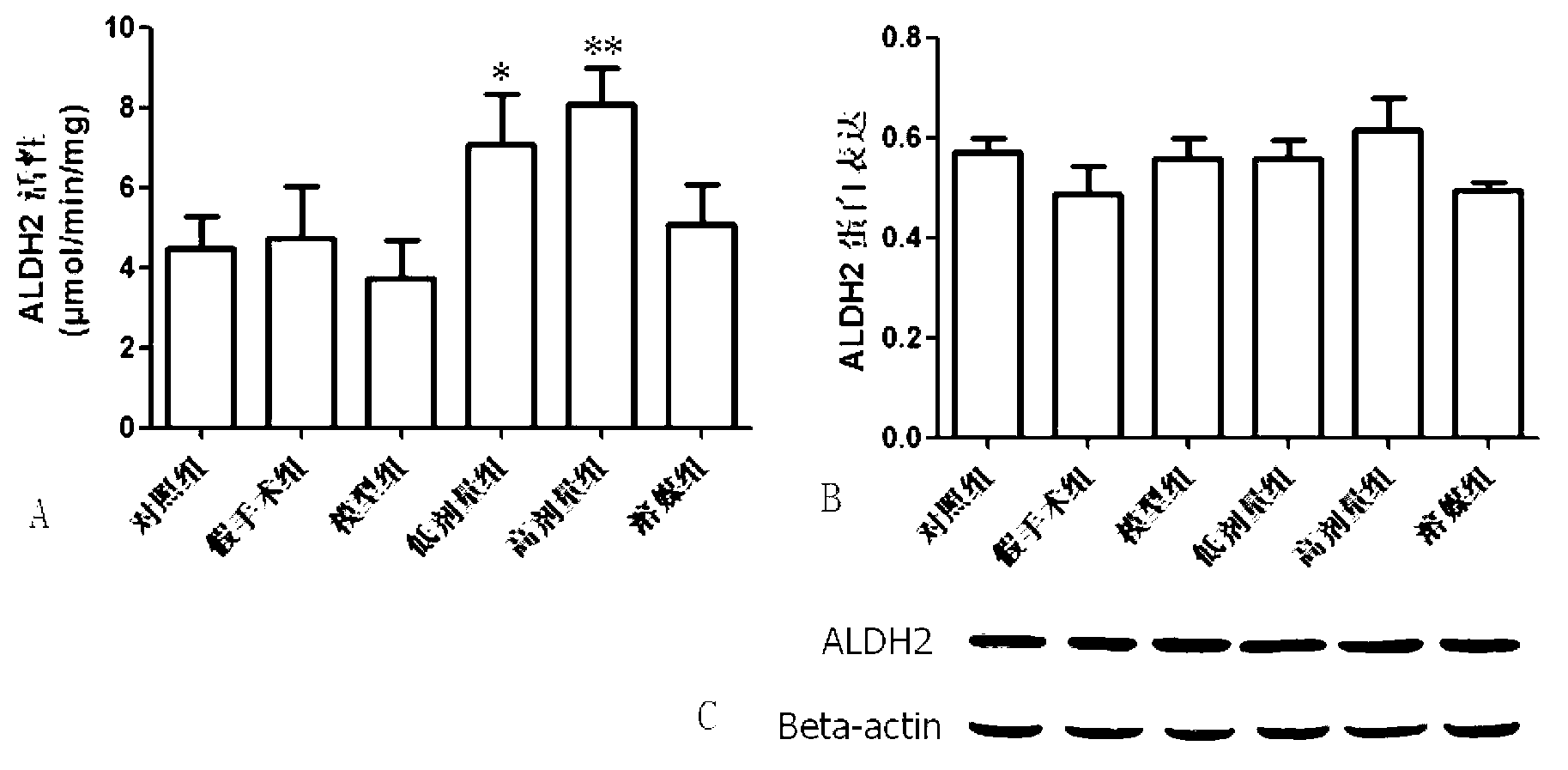
[0039] Protective effect of Alda-1 on cerebral ischemia-reperfusion injury
[0040] Implemented drugs: carboxymethylcellulose sodium (CMC-Na) was purchased from Shanghai Shanpu Chemical Co., Ltd.
[0041] The alda-1 compound prepared in Example 1 was prepared into a suspension using 0.5% carboxymethylcellulose so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com