Preparation method of polyether diol
A technology of polyether diol and polyether monool, which is applied in the field of preparation of polyether diol, and achieves the effects of cheap and easy-to-obtain raw materials, high yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Preparation of compound (3)
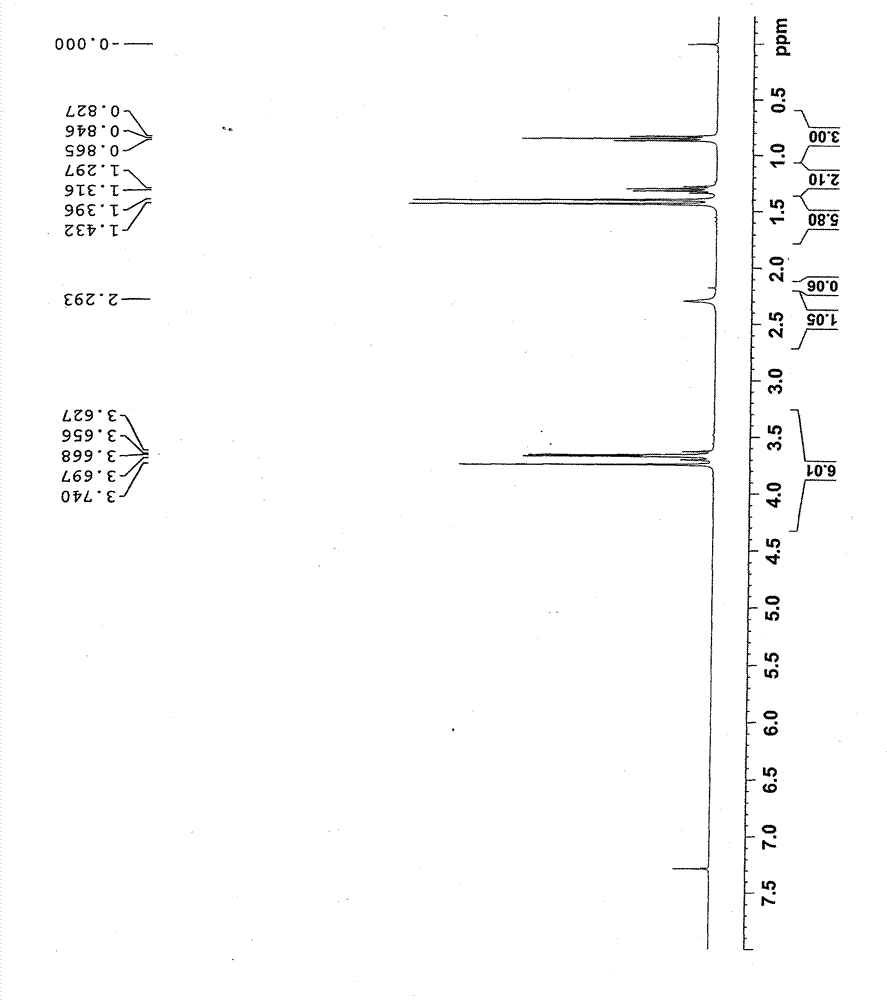
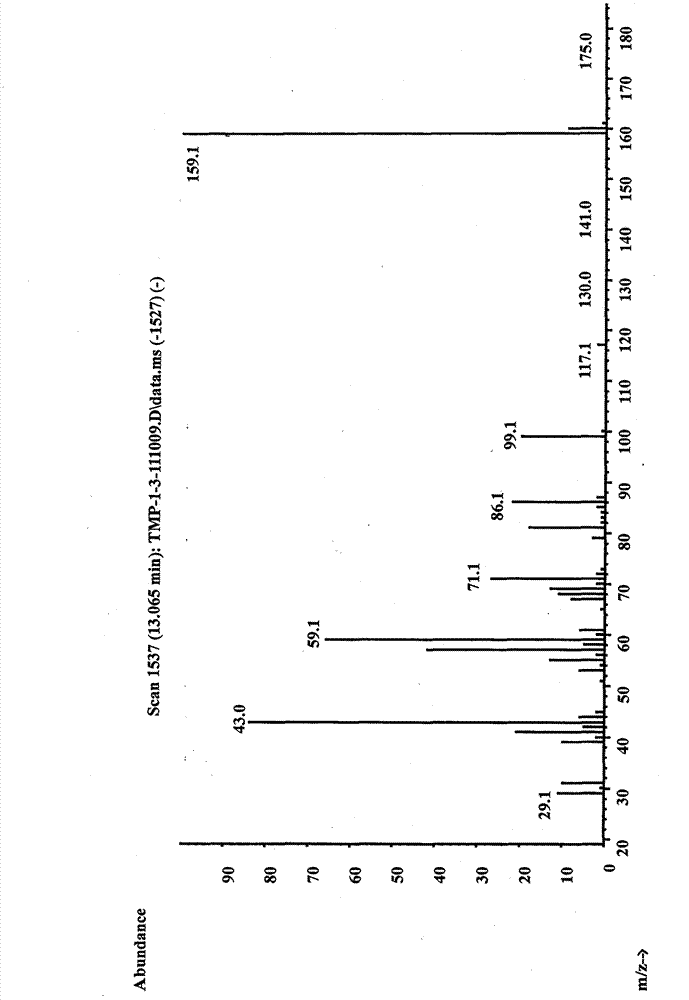
[0038] Add 268g of trimethylolpropane, 928g of acetone and 9.16g of ferric sulfate into a three-necked flask, raise the temperature to 56°C, and keep the reaction for 7 hours to obtain a yellow reaction solution. Add potassium acetate aqueous solution to the reaction solution to adjust the pH value to approximately equal to 7, and then collect the yellow filtrate by filtration; use dichloromethane to perform liquid-liquid extraction on the filtrate, collect the dichloromethane phase, dry it over anhydrous sodium sulfate, and filter it at 45°C Distillation under reduced pressure (vacuum degree: 0.098 MPa) gave 320 g of a light yellow transparent liquid with a yield of 92.0%. Such as figure 1 and 2 As shown, the product was determined to be 2,2-dimethyl-1,3-dioxane-5-ethyl-5-methanol by GC and NMR analysis.
[0039]The gas phase analysis conditions are: Shimadzu 2010 gas chromatograph (FID detector), chromatographic column type DB-5, c...
Embodiment 2
[0054] (1) Preparation of compound (3)
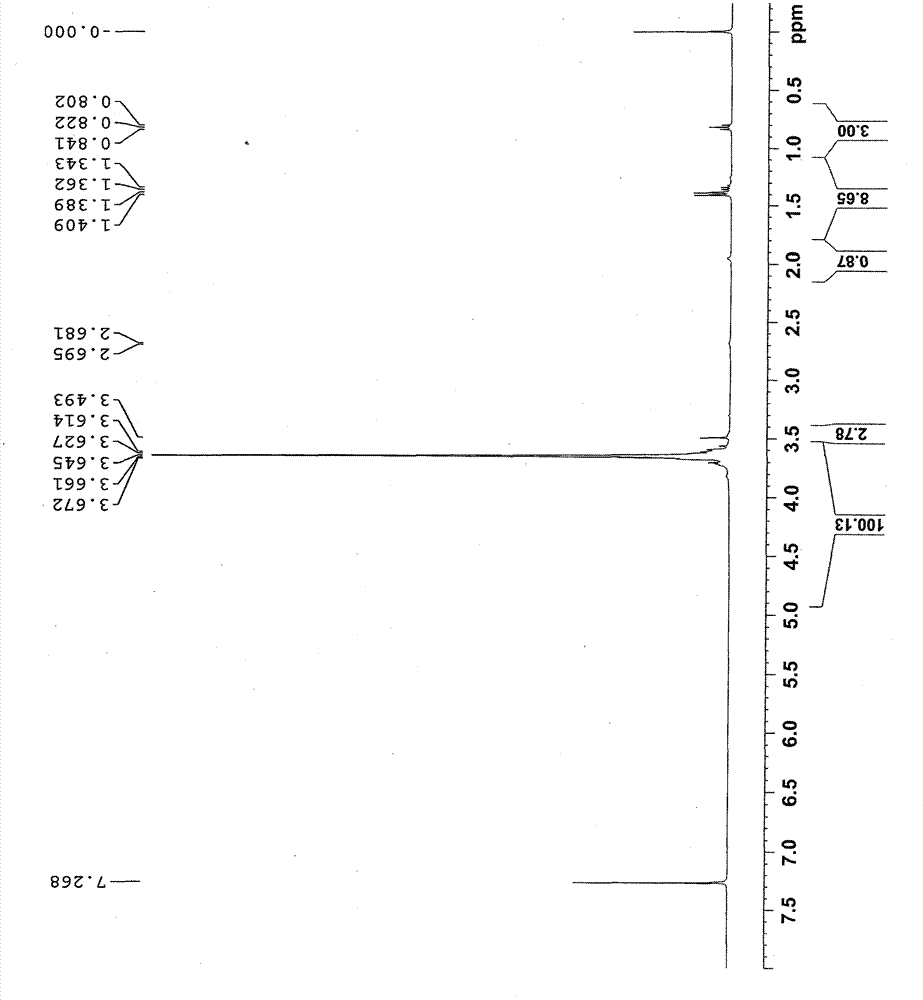
[0055] Add 268g of trimethylolpropane, 696g of acetone and 2.15g of strong acidic resin into a three-necked flask, raise the temperature to 56°C, and keep the reaction for 7h. The reaction solution was filtered to obtain a colorless transparent solution, which was distilled under reduced pressure at 45°C (vacuum degree: 0.098MPa) to obtain 311g of a light yellow transparent liquid, namely the compound 2,2-dimethyl-1,3-dioxane-5- Ethyl-5-methanol, the yield is 89.5%, and the product purity is 99.3%.
[0056] The product structure is:
[0057]
[0058] (2) Preparation of polyether monoalcohol (4)
[0059] Accurately weigh 1063g of ethylene oxide and put it into a storage tank, put 87g of compound (3) and 5.75g of KOH into a 2L reaction kettle, replace the air with nitrogen three times, then heat up to 95°C for vacuum dehydration for 1h (vacuum degree is 0.098 MPa), then start to add ethylene oxide, keep the temperature in the kettle...
Embodiment 3
[0067] (1) Preparation of compound (3)
[0068] Add 268g of trimethylolpropane, 464g of acetone and 6.7g of acid clay into a three-necked flask, raise the temperature to 45°C, and keep the temperature for 5h. The reaction solution was filtered to obtain a colorless and transparent solution, which was distilled under reduced pressure at 45°C (vacuum degree of 0.098MPa) to obtain 295g of a light yellow transparent liquid, namely the compound 2,2-dimethyl-1,3-dioxane-5- Ethyl-5-methanol, the yield is 84.7%, and the product purity is 99.6%.
[0069] The product structure is:
[0070]
[0071] (2) Preparation of polyether monoalcohol polyether monoalcohol (4)
[0072] Accurately weigh 1663g ethylene oxide and put it into the storage tank, and 87g compound (3) and 8.75gBa(OH) 2 Put it into a 2L reaction kettle, replace the air with nitrogen three times, then heat up to 95°C for vacuum dehydration for 1h (vacuum degree is 0.098MPa), then start adding ethylene oxide, keep the te...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydroxyl value | aaaaa | aaaaa |
| hydroxyl value | aaaaa | aaaaa |
| hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com