Preparation method of aggregation-induced emission molecule
A technique of aggregation-induced luminescence and molecules, applied in luminescent materials, chemical instruments and methods, and the preparation of amino compounds from amines, etc., can solve problems such as complex synthesis processes, achieve high fluorescence quantum yield, high yield, and good thermal stability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
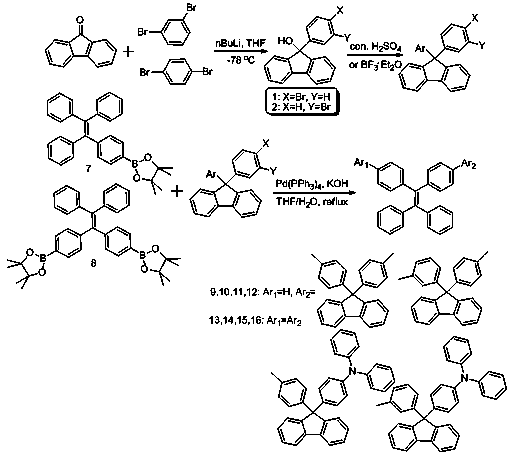
[0020] The synthesis of embodiment 1 compound 9-16
[0021] Synthetic roadmap as figure 1 shown.
[0022] (1) Under nitrogen or argon atmosphere, 1,4-dibromobenzene (4.72g, 20.0mmol) was dissolved in 60mL of anhydrous tetrahydrofuran (THF) and cooled at -78°C for half an hour. To the above solution, 9.2 mL of n-butyl lithium in n-hexane (n-BuLi, 2.3 M) was added dropwise. After low-temperature reaction for 1 hour, 50 mL of fluorenone (3.61 g, 20 mmol) in tetrahydrofuran (THF) was added, and the low-temperature reaction was continued for 3 hours, then it was allowed to rise to room temperature naturally, and stirred overnight. After the reaction, water was added to the reaction liquid, extracted with chloroform, and the organic phase was collected and dried over anhydrous sodium sulfate. Using petroleum ether and chloroform (v / v, 4 / 1) as the eluent, the product was chromatographed on a silica gel column, separated and purified, and dried in vacuo to obtain a pale yellow oil ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com