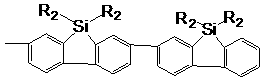
2,2',7,7'-spirosilabifluorene oligomer, and preparation method and application thereof
A kind of technology of oligomer and tetrabromospirosilicofluorene, applied in the field of electroluminescent materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] according to Eur. Polym. J. 2010, 46, 2365-2371 disclosed method to prepare 2-(4-biphenyl)-7-tributyltin-9,9-dioctylfluorene.
[0041] The synthetic route is as follows:
[0042]
[0043]
[0044] (1) 2,7-dibromo-9,9-dioctylfluorene
[0045] Add 22.5g of fluorene and 150ml of chloroform into a 250ml round bottom flask, cool to 0°C, add 0.01g of iron powder, and then add 12ml of liquid bromine. Reacted for 3 hours, filtered, and concentrated chloroform to obtain 21.9 g of white crystals, with a yield of 50%. Add 16.2g of 2,7-dibromofluorene, 0.161g of tetrabutylammonium bromide, 250ml of DMSO, 12ml of 50% sodium hydroxide solution into a 500ml round bottom flask, and then dropwise add 19.3g of 1-bromo-n-octane. After reacting for 3 hours, stop the reaction. Add saturated brine, extract with anhydrous ether, combine organic layers, and dry over anhydrous magnesium sulfate. Filtration, rotary evaporation, petroleum ether / silica gel column, 19.9g of white ...
Embodiment 2
[0056] Example 2 Synthesis of 2,2',7,7'-tetrabromospirosilafluorene
[0057] according to J. Am. Chem. Soc. , 2005, 127, 7662–7663 to prepare 2,2'-diiodo-4,4'-dibromobiphenyl by the method disclosed. The synthetic route is as follows:
[0058]
[0059] (1) 2,2’-Dinitro-4,4’-dibromobiphenyl
[0060] Add 50g of 2,5-dibromonitrobenzene, 27.8g of copper powder, and 180ml of DMF into a 500ml three-neck flask, heat to 120°C and react for 3 hours, stop the reaction, cool to room temperature, add 240ml of toluene, stir for 30 minutes, filter , and the filtrate was washed with saturated brine and water. The organic layers were combined, dried over anhydrous magnesium sulfate, filtered, evaporated to dryness, and recrystallized from absolute ethanol to obtain 31.5 g of a light yellow solid with a yield of 88%.
[0061] 1H-NMR (CDCl 3 , 500MHz,ppm) 8.37 (d, 2H), 7.82 (m, 2H), 7.15 (d, 2H).
[0062] (2) 2,2'-diiodo-4,4'-dibromobiphenyl
[0063]In a 250ml flask, add 18g ...
Embodiment 3
[0068] Example 3 Synthesis of 2,2',7,7'-tetrakis[2-(4-biphenyl)-9,9-dioctylfluorenyl]spirosilafluorene
[0069]
[0070] In a 100ml round bottom flask, add 0.15g 2,2'7,7'-tetrabromospirosilafluorene, 1.34g 2-(4-biphenyl)-7-tributyltin-9,9-dioctylfluorene, 32.3 mg Pd(PPh 3 ) 2 Cl 2 , 60ml THF, heated to 70°C, refluxed for 72 hours, and stopped the reaction. Add water, extract with dichloromethane, combine organic layers, and dry over anhydrous magnesium sulfate. Filter and evaporate. The n-hexane / silica gel was passed through the column to obtain 0.3 g of a yellow solid with a yield of 44%.
[0071] 1H-NMR (CDCl 3 , 500MHz,ppm) 7.76-7.75 (t, 2H), 7.74-7.73 (d, 2H), 7.70 (s, 3H), 7.68 (s, 1H), 7.66 (s, 1H), 7.64 (s, 2H) , 7.62 (d, 1H), 7.60 (t, 1H), 7.58 (s, 2H), 7.48-7.44 (t, 4H), 7.35-7.33 (t, 2H), 7.31 (d, 2H), 7.29-7.27 (d, 2H), 2.01-1.97 (m, 6H), 1.35-0.77 (m, 24H), 1.18-1.04 (m, 38H), 0.92-0.85 (m, 18H), 0.80-0.77 (m, 14H) , 0.70-0.63 (m, 10H).
[0072] Elem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com