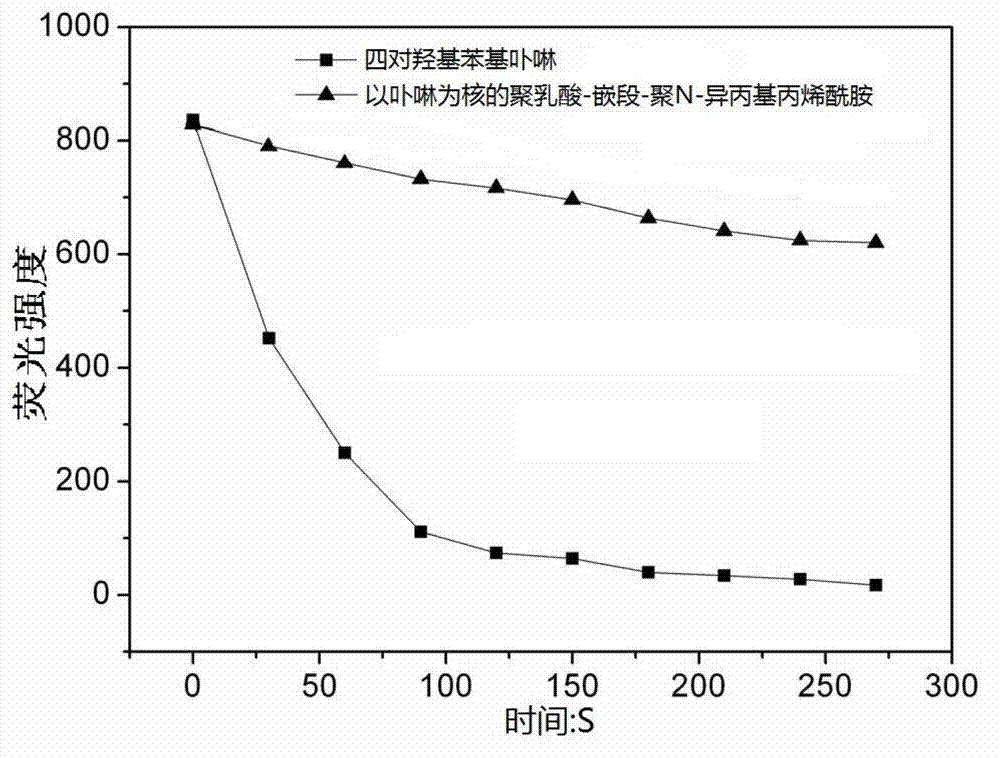
Method for synthesizing polylactic acid-block-polyN-isopropyl acrylamide temperature-sensitive material
A technology of isopropylacrylamide and temperature-sensitive materials, which is applied in the fields of wave energy or particle radiation treatment materials, drug combinations, pharmaceutical formulations, etc. N-isopropylacrylamide preparation method and other problems, to achieve the effect of mild reaction and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
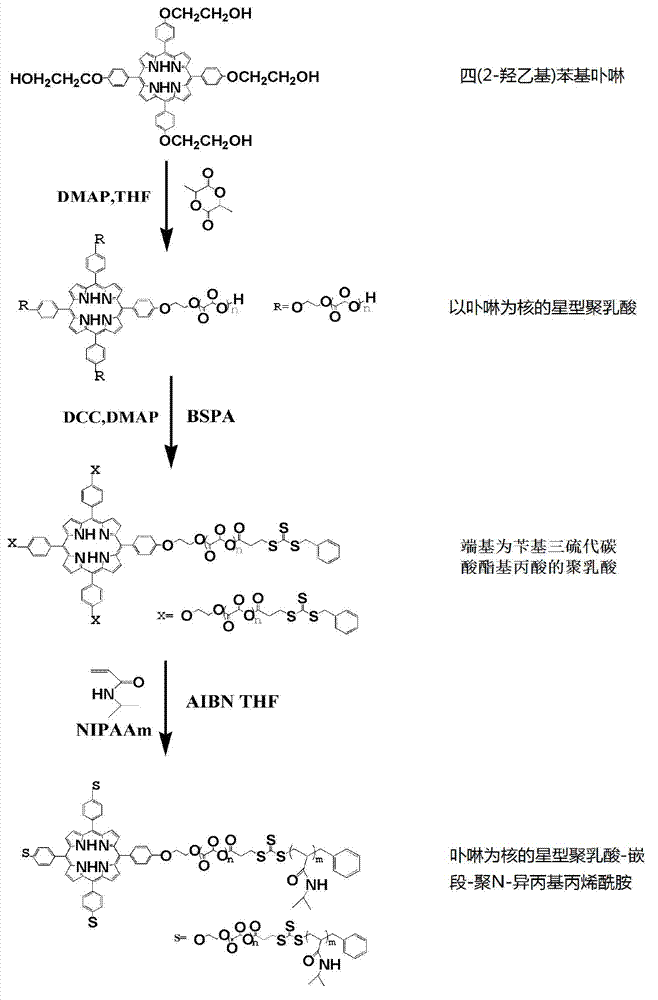
[0022] Embodiment 1: The preparation method of star-shaped polylactic acid-block-poly N-isopropylacrylamide temperature-sensitive material with porphyrin as the core
[0023] Tetrakis(2-hydroxyethyl)phenylporphyrin (68.4mg, 0.08mmol) was used as the initiator, DMAP (78.2mg, 0.64mmol), L-lactide (922mg, 6.4mmol) were successively added to the fully dried In the test tube, seal it with an inversion plug. After the vacuum line is operated and evacuated nitrogen for three times, add 0.2mLTHF and put it in a constant temperature oil bath at 50°C. After reacting for 24h, cool the test tube to room temperature, and dissolve the obtained solid in 5mLCH 2 Cl 2 , and settled dropwise in 50 mL of ice methanol under magnetic stirring. The product obtained after filtration was vacuum-dried at 40° C. to constant weight. Yield 931.7 mg, 87.2% yield.
[0024] Add dried star polylactic acid (SPPLA-OH) (110.4 mg, 0.01 mmol) and benzyl trithiocarbonate propionic acid (BSPA) (21.76 mg,...
Embodiment 2
[0026] Embodiment 2: The preparation method of star-shaped polylactic acid-block-poly N-isopropylacrylamide temperature-sensitive material with porphyrin as the core
[0027] The preparation of star polylactic acid (SPPLA-OH) and macromolecular chain transfer agent SPPLA-BSPA with porphyrin as the nucleus terminal hydroxylation is the same as in Example 1.
[0028] Add SPPLA-BSPA (20mg, 0.002mmol), NIPAAm (43.1mg, 0.4mmol) and AIBN (0.2mg, 0.0016mmol) into a 25ml round bottom flask, then add 0.8ml of THF to fully dissolve them. After evacuating nitrogen three times with a vacuum line, react in a constant temperature oil bath at 70° C. for 12 hours. After the reaction, cool in an ice-water bath, settle in anhydrous ether, centrifuge, take the solid, dissolve it in THF, settle with n-hexane, remove unreacted NIPAAm monomer, and centrifuge to obtain a solid product. Drying in vacuo at constant temperature to constant weight yielded 48.6 mg star-shaped polylactic acid-b...
Embodiment 3
[0029] Example 3: The preparation method of the star-shaped polylactic acid-block-poly N-isopropylacrylamide temperature-sensitive material with porphyrin as the core
[0030] The preparation of star polylactic acid (SPPLA-OH) and macromolecular chain transfer agent SPPLA-BSPA with porphyrin as the nucleus terminal hydroxylation is the same as in Example 1.
[0031] Add SPPLA-BSPA (20mg, 0.002mmol), NIPAAm (86.9mg, 0.8mmol) and AIBN (0.2mg, 0.0016mmol) into a 25ml round bottom flask, and then add 0.8ml of THF to fully dissolve them. After evacuating nitrogen three times with a vacuum line, react in a constant temperature oil bath at 70° C. for 12 hours. After the reaction, cool in an ice-water bath, settle in anhydrous ether, centrifuge, take the solid, dissolve it in THF, settle with n-hexane, remove unreacted NIPAAm monomer, and centrifuge to obtain a solid product. Vacuum drying at constant temperature to constant weight yielded 87.9 mg star-shaped polylactic acid-bl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com