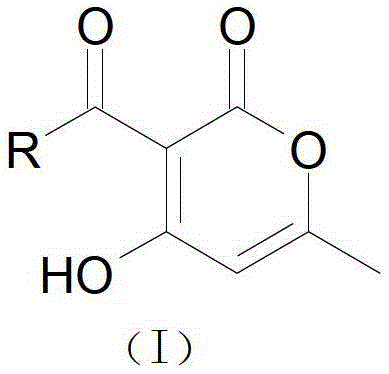
3-substituted alpha-pyrone derivative as well as preparation method and application thereof
A technology of pyrone and preparation, applied in the field of agricultural fungicides, can solve the problems of high price and harsh experimental conditions, and achieve the effects of low cost, high bacteriostatic rate and obvious biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
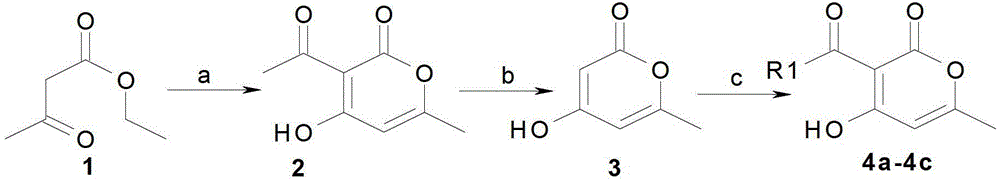
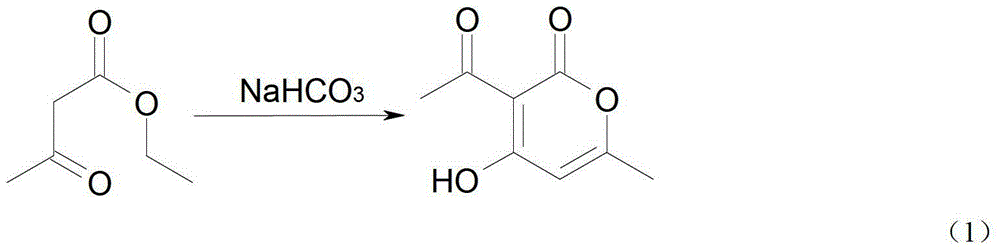
[0036] Synthesis of Example 13-acetyl-4-hydroxyl-6-methyl-2-pyrone (formula 2)
[0037] Heat 100g (780mmol) of freshly distilled ethyl acetoacetate and 0.5g of sodium bicarbonate to 200-210°C and keep heating for 7 hours. During the heating process, the distillate at 72°C will evaporate, and about 27g (mostly ethanol) of the reactant will change color It was dark brown, distilled under reduced pressure, collected the fraction at 140°C and 12mm, and obtained a crude product, 34g (53%) mp.104~110°C, recrystallized from ethanol, and obtained 3-acetyl-4-hydroxy-6-methanol Pure 2-pyrone 27g, m.p.110~111℃;
[0038] H-NMR (300MHz, CDCl3): 2.271 (d, 3H, CH3), 2.666 (s, 3H, CH3), 5.935 (s, 1H, Ar-CH).
[0039] The structural formula is as follows:
[0040]
Embodiment 2
[0041] The synthesis of embodiment 26-methyl-4-hydroxyl-2-pyrone (formula 3)
[0042]3-acetyl-4-hydroxy-6-methyl-2-pyrone 50g (290mmol) prepared by the method of Example 1 was dissolved in 150g mass concentration of 90% H 2 SO 4 solution, heated to 130°C and maintained for 15 minutes, the reaction flask was cooled rapidly, the reactant was poured into 200 ml of ice-cold water, the precipitate was filtered with suction, washed with 2×15 ml of cold water, and dried to obtain 6-methyl-4-hydroxy- 2-Pyrone, the product is a white solid, 36g (90%), mp.188-189°C. 1H NMR (CDCl 3 ) chemical shift: 2.245 (s, 3H, CH 3 ),5.484(d,1H,CH),5.885(s,1H,CH),1.557(s,1,H 2 O, weighted water disappears). HR-MS (ESI) m / z 127.0398 (M+H+, 100%). The structural formula is as follows:
[0043]
Embodiment 33
[0044] Synthesis of Example 33-propionyl-4-hydroxyl-6-methyl-2-pyrone (formula 4a)
[0045] 6-methyl-4-hydroxyl-2-pyrone (0.5g, 0.0040mol), propionic acid (0.30g, 0.0041mol) dicyclohexylcarbodiimide (DCC) prepared by the method of Example 2 (0.90g, 0.0045mol) and 4-dimethylaminopyridine (DMAP) (0.1g, 0.00082mol) were added to a 100ml round-bottom flask, and 50ml of toluene was added to dissolve it, and stirred evenly. Heat and stir the reaction in an oil bath at 100°C for 6 hours, and detect the reaction by spotting a plate, and there is one main fluorescent point. After the reaction was completed, remove most of the solvent with a rotary evaporator, add 100ml ethyl acetate, wash three times with 50ml of water, dry the organic phase with anhydrous sodium sulfate, filter with suction, then concentrate by rotary distillation, and separate by column chromatography (mobile phase is E:P =1:6) to obtain 3-propionyl-4-hydroxy-6-methyl-2-pyrone. Yield 50~60%, mp.101~102℃. H-NMR (30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com