Preparation methods of cyclodextrin-based star-block polymer and cyclodextrin-based star-block polymer/gold nano-rod composite material
A technology of block polymer and cyclodextrin, which is applied in the field of preparation of cyclodextrin-based star-shaped block polymer and cyclodextrin-based star-shaped block polymer/gold nanorod composite material. The effect of controllable shape, clear structure and simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] A preparation method of cyclodextrin-based star block polymer, comprising the following steps:
[0029] 1) Combine β-cyclodextrin, P 2 S 5 , p-methoxybenzoic acid three in K 2 CO 3 Reflux reaction in the organic solvent DMF for 16-18h in the presence and anaerobic conditions, purify the obtained product to obtain dithioester, and obtain RAFT polymer chain transfer agent (CD-CTA);
[0030] 2) Mix the RAFT polymer chain transfer agent prepared in the previous step, methyl methacrylate, and functional monomers, and carry out living free radical polymerization in the solvent DMF in the presence of free radical initiators and under anaerobic conditions, and ice-water bath Cooling stops the polymerization, and the resulting product is purified and separated;
[0031] 3) The product obtained in the above steps is used as a macromolecular chain transfer agent. In the presence of a free radical initiator and under oxygen-free conditions, the hydrophilic monomer and the macro...
Embodiment 1
[0040] Example 1: Synthesis of RAFT polymeric chain transfer agent (CD-CTA):
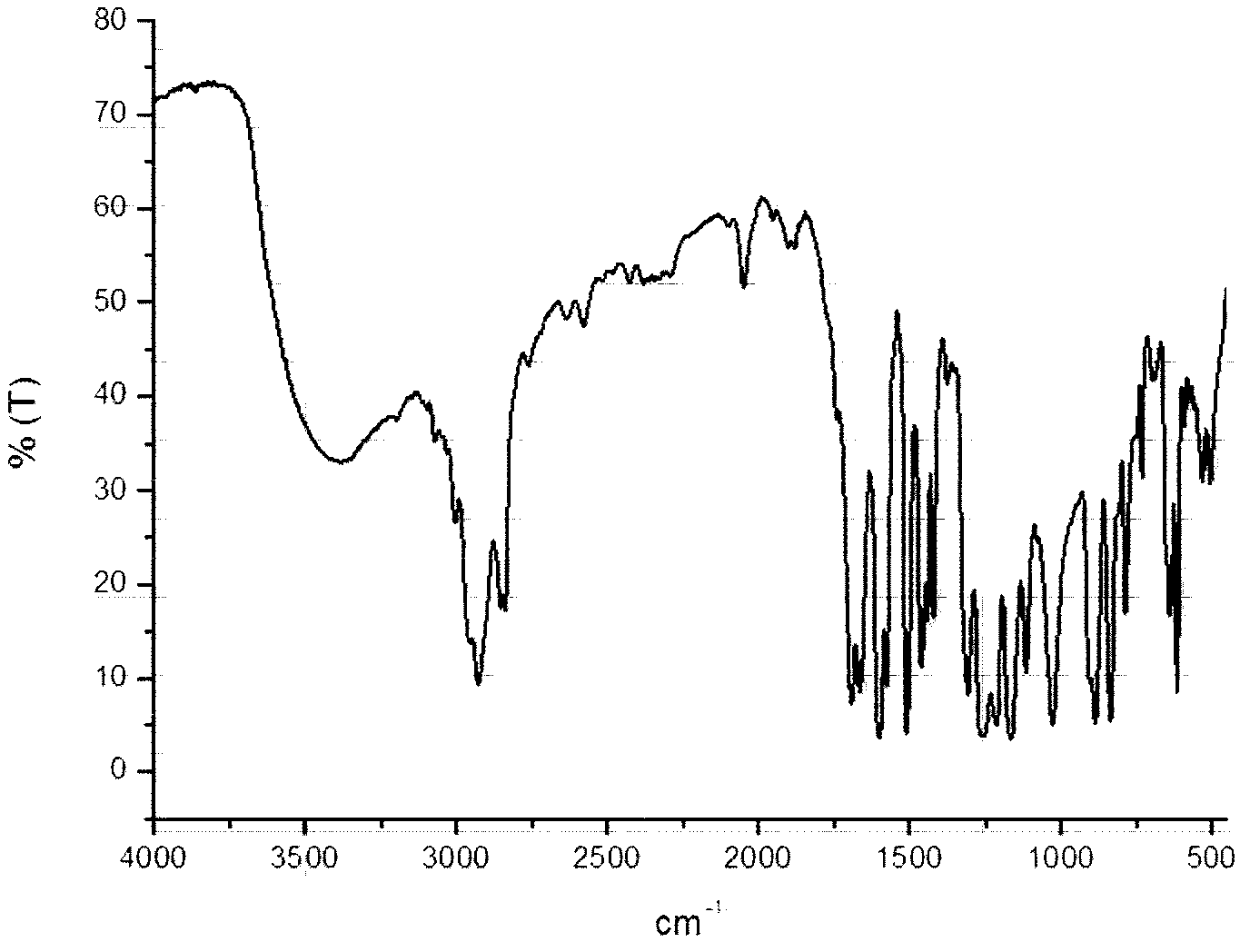
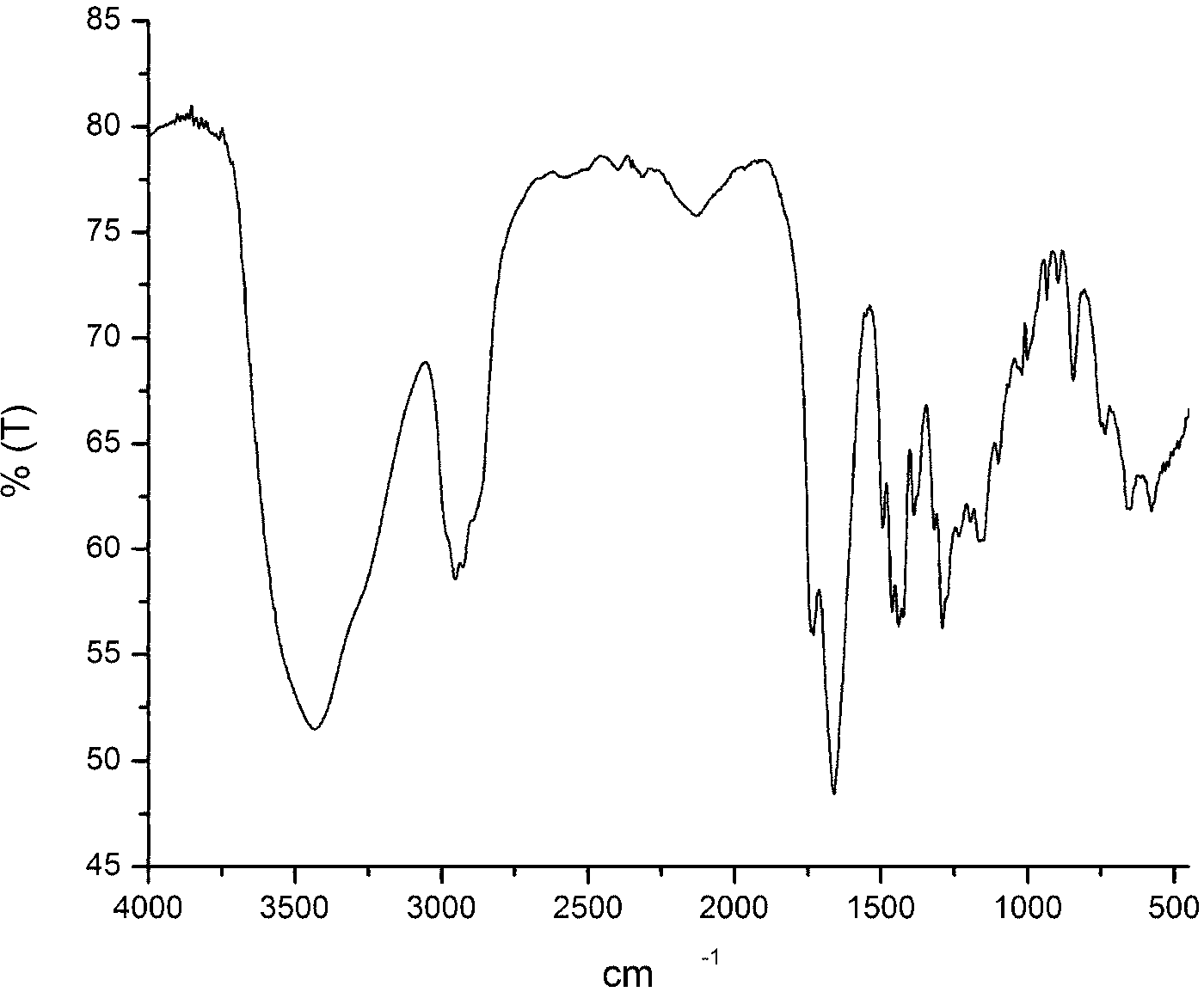
[0041] Into a 250 ml three-necked bottle, add metered 10mmol β-CD (ie β-cyclodextrin), 70mL DMF, 30mmol P 2 S 5 , 1.5g K 2 CO 3 And 30mmol of p-methoxybenzoic acid, passed through Ar and stirred for 15 minutes, then refluxed for 16 hours, and finally a reddish-brown suspension was obtained. Wash with deionized water (3*50mL), extract with ethyl acetate (2*30mL), spin dry and pass through a silica gel column. The mobile phase used is a mixture of petroleum ether:ethyl acetate (volume ratio)=20:1 , collect the orange-red fraction, and recrystallize from ethyl acetate to obtain the orange-red solid product CD-CTA, with a yield of 30%. FI-IR: 1069.5cm -1 (S=C-S-), 1390~1560 cm -1 (-C 6 h 4 -); H-NMR: 7.01~7.53 (-C 6 h 4 -); 2.75~3.60 (β-CD). like figure 1 Infrared spectrum of the RAFT polymeric chain transfer agent (CD-CTA) synthesized in this example.
Embodiment 2
[0042] Embodiment 2: the synthesis of star block copolymer
[0043] CD-[P(A-co-B)] n Synthesis: A is methyl methacrylate, B is hydroxyethyl acrylate
[0044] According to the molar ratio of Monomer(A+B):CD-CTA:AIBN=1000:5:1 (A:B=6:1, molar ratio), DMF (10~15ml) was used as solvent, argon gas was passed for 15min, and then Vacuum degassing, vacuum fusion sealing after 3 cycles. React under magnetic stirring at 80°C for 12 hours, then cool in an ice-water bath to stop polymerization, use methanol as a precipitant, dissolve with DMF after suction filtration, dissolve-precipitate twice, and then dialyze for 72 hours with a dialysis bag with a molecular weight cut-off of 7000Da. After vacuum drying, light yellow solid CD-[P(A-co-B)] was obtained n .
[0045] CD-[P(A-co-B-b-C)] n Synthesis of: the C monomer described below is N-hydroxypropyl acrylamide;
[0046] CD-[P(A-co-B)] synthesized above n It is a macromolecular chain transfer agent (Macro-CTA) with a molar ratio of Mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com