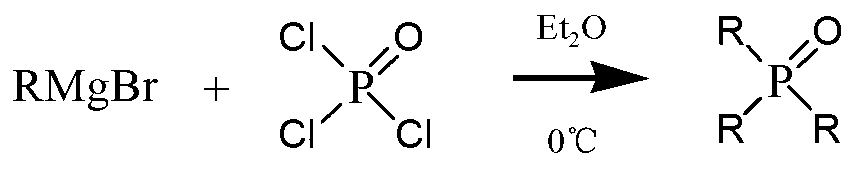
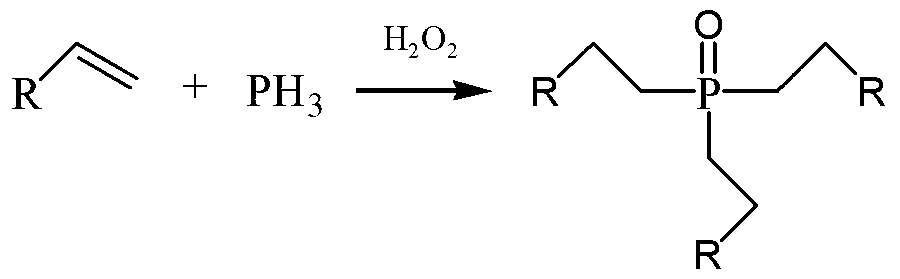
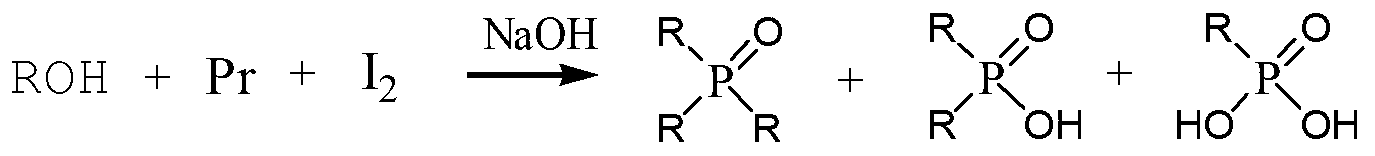
Trioctylphosphine oxide compound and preparation method thereof
A technology of trioctyl phosphorus oxide and compound, applied in the field of trioctyl phosphorus oxide compound and preparation thereof, can solve the problems of unfavorable industrial application, low yield and high cost, and achieves easy control, convenient purification and enhanced safety. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The synthesis of trioctyl phosphorus oxide, taking n-octene as an example, includes the following steps:
[0051] 1) Octene addition:
[0052] Add 10g (0.114mol) of sodium hypophosphite to 50ml of acetic acid. After heating to 120°C, slowly add 30g (0.268mol) of n-octene and continue heating to 135°C for 4 hours. 31 PNMR detects the end of the reaction. After the reaction is complete, cool, add water and chloroform to separate liquids to remove acid, vacuum distillation (400Pa, 35℃) to remove organic solvents, vacuum drying (120Pa, 25℃) to obtain white solid dioctylphosphine Acid 30.8g (0.106mol). The yield was 93.2%.
[0053] 2) Trichlorosilane reduction
[0054] Put 2g (0.007mol) of dioctylphosphinic acid obtained in step 1 into a 50ml airtight reactor, add 30ml of chloroform (dehydrated) as a solvent, add 2g (0.015mol) of trichlorosilane, and react at 80°C 16 hours, 31 The end point of the reaction was detected by PNMR. After the reaction was completed, the organic solvent...
Embodiment 2
[0060] The difference from Example 2 is the process after step 2.
[0061] 1) Hydrolysis: Dissolve 2g (0.007mol) of dioctylphosphorus chloride obtained in step 2 of Example 1 in 20ml of chloroform, add distilled water to hydrolyze, 31 The end of the reaction was detected by PNMR. After the reaction was completed, lye was added for liquid separation, the water layer was removed, chloroform was distilled off under reduced pressure (400Pa, 35°C), and dried under vacuum to obtain 1.73g (0.006mol) of white solid dioctylphosphonic acid. The yield was 92.9%.
[0062] 2) Addition of octene again: Put 2g of dioctylphosphonic acid (0.007mol) into 20ml of acetic acid, heat to 120°C, slowly drop in 2.2 (0.020mol)g of octene, and continue heating to 135°C for 8 hours , 31 PNMR detects the end of the reaction. After the reaction is complete, add chloroform and distilled water to remove acetic acid, distill under reduced pressure to remove chloroform (400Pa, 35°C), vacuum dry (120Pa, 25°C) to obt...
Embodiment 3
[0064] Dissolve 2g (0.007mol) of dioctylphosphorus chloride obtained in step 2 of Example 1 in 20ml of chloroform, add 1g (0.0077mol) of n-octanol, and react at 50°C for 2 hours. 31 The end point of the reaction was detected by PNMR. After the reaction was completed, water was added and the chloroform (400Pa, 35°C) and excess n-octanol (400Pa, 65°C) were removed by distillation under reduced pressure to obtain 2.57g (0.0067 mol). Yield 95.1%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com