Preparation method of letrozole
A technology of letrozole and its intermediates, applied in the field of preparation of aromatase inhibitors, can solve the problems of cumbersome process operation, high operation risk, long cycle, etc., achieve high yield and purity, simplify the process, and avoid yield low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
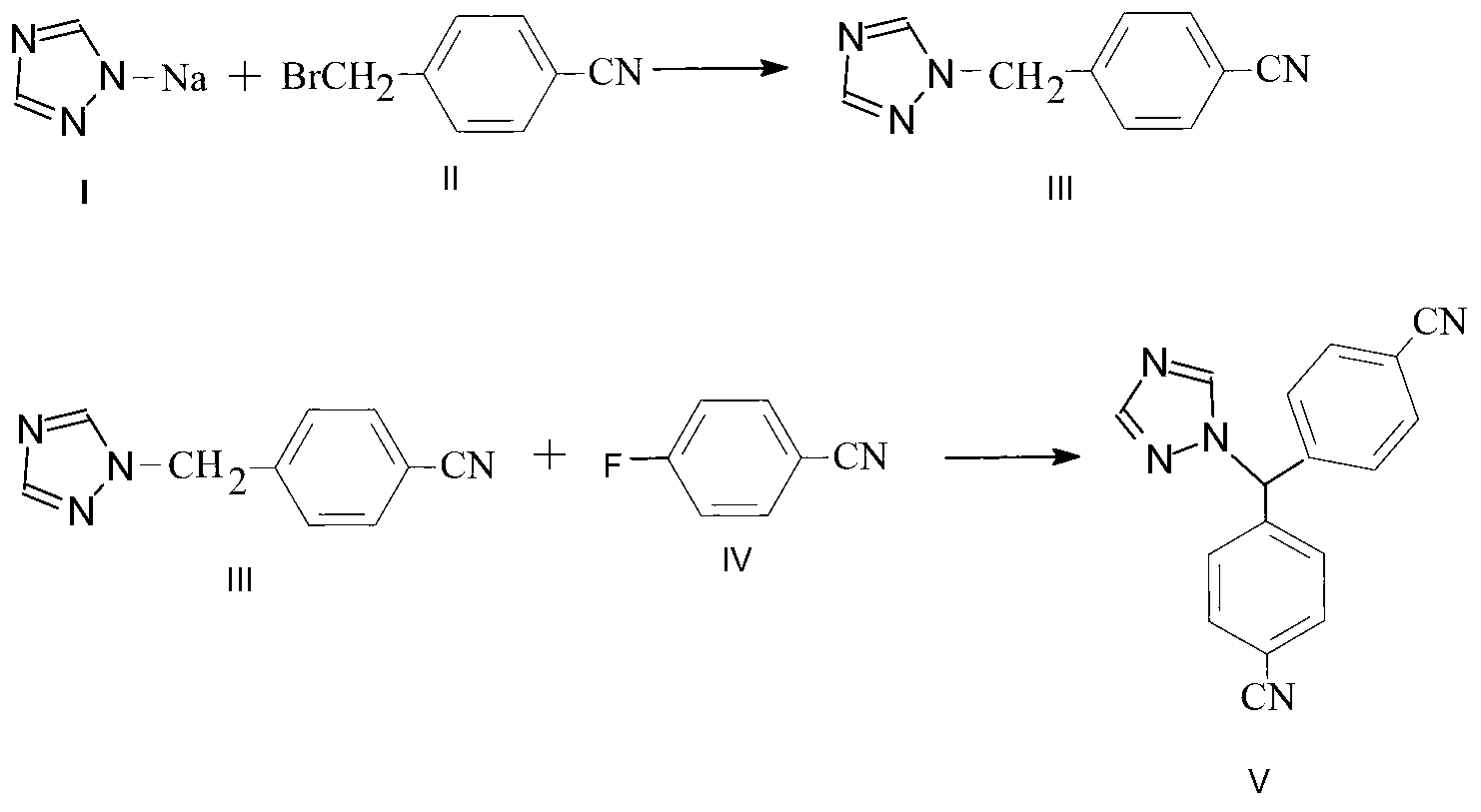
[0024] (1) Preparation of letrozole intermediate III, 4-[1H-(1,2,4-triazolyl)methyl]benzonitrile
[0025] Add 31.4g of compound I, 1,2,4-triazole sodium salt and 400ml of dimethyl sulfoxide solvent in a 1L reaction flask, add 40g of compound II, 4-bromomethylbenzonitrile at room temperature, and react 2 hours. After the reaction, extract the organic phase with 3N hydrochloric acid for 3 times, combine the water phase, add sodium carbonate solution to the water phase for crystallization, then grow the crystal at 0°C for 1.5 to 2 hours, and obtain 30 g of solid letrozole intermediate III by suction filtration. The molar yield is 80%, and the HPLC purity is 99.2%.
[0026] (2) Preparation of Letrozole
[0027] Add 3.84g of potassium tert-butoxide and 28ml of dimethyl sulfoxide solvent to a 250ml four-necked bottle, and lower the temperature to -30°C with liquid nitrogen; use 2g of intermediate III, 1.4g of p-fluorobenzonitrile and 24ml of dimethyl Prepare the intermediate-p-fl...
Embodiment 2
[0030] (1) Preparation of letrozole intermediate III, 4-[1H-(1,2,4-triazolyl)methyl]benzonitrile
[0031] In a 1L reaction flask, add 62.8g compound I, 1,2,4-triazole sodium salt and 1200ml of N-methylpyrrolidone solvent, add 80g compound II, 4-bromomethylbenzonitrile at room temperature, and react 1 hour. After the reaction, use 3N hydrochloric acid to extract the organic phase for 3 times, combine the water phase, add sodium bicarbonate solution to the water phase for crystallization, then grow the crystal at 0°C for 1.5-2 hours, and filter with suction to obtain 61g of solid letrozole intermediate III , molar yield 81.3%, HPLC purity 99.5%.
[0032] (2) Preparation of Letrozole
[0033] Add 3g of potassium tert-butoxide and 40ml of N-methylpyrrolidone solvent into a 250ml four-necked bottle, and lower the temperature to -32°C with liquid nitrogen; use 2g of intermediate III, 1.4g of p-fluorobenzonitrile and 35ml of N-methyl Prepare the intermediate-p-fluorobenzonitrile s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com