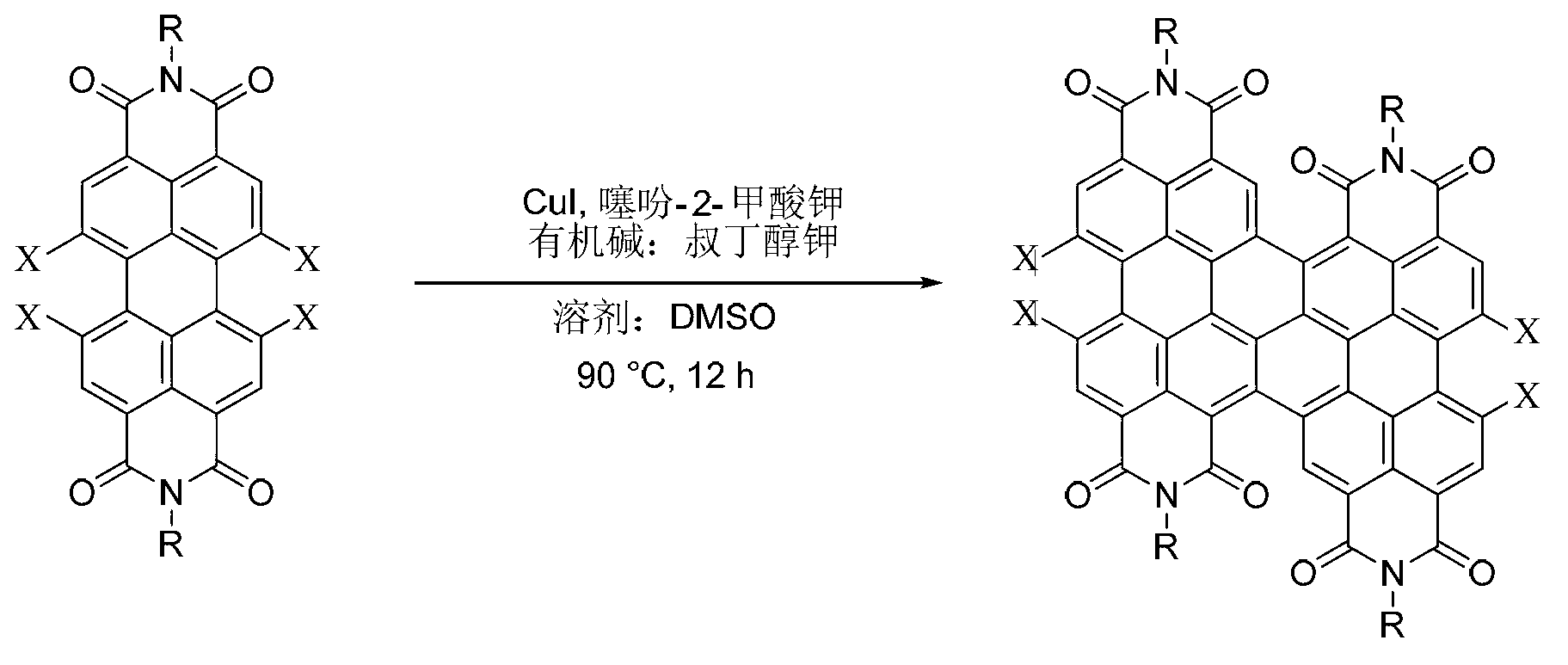
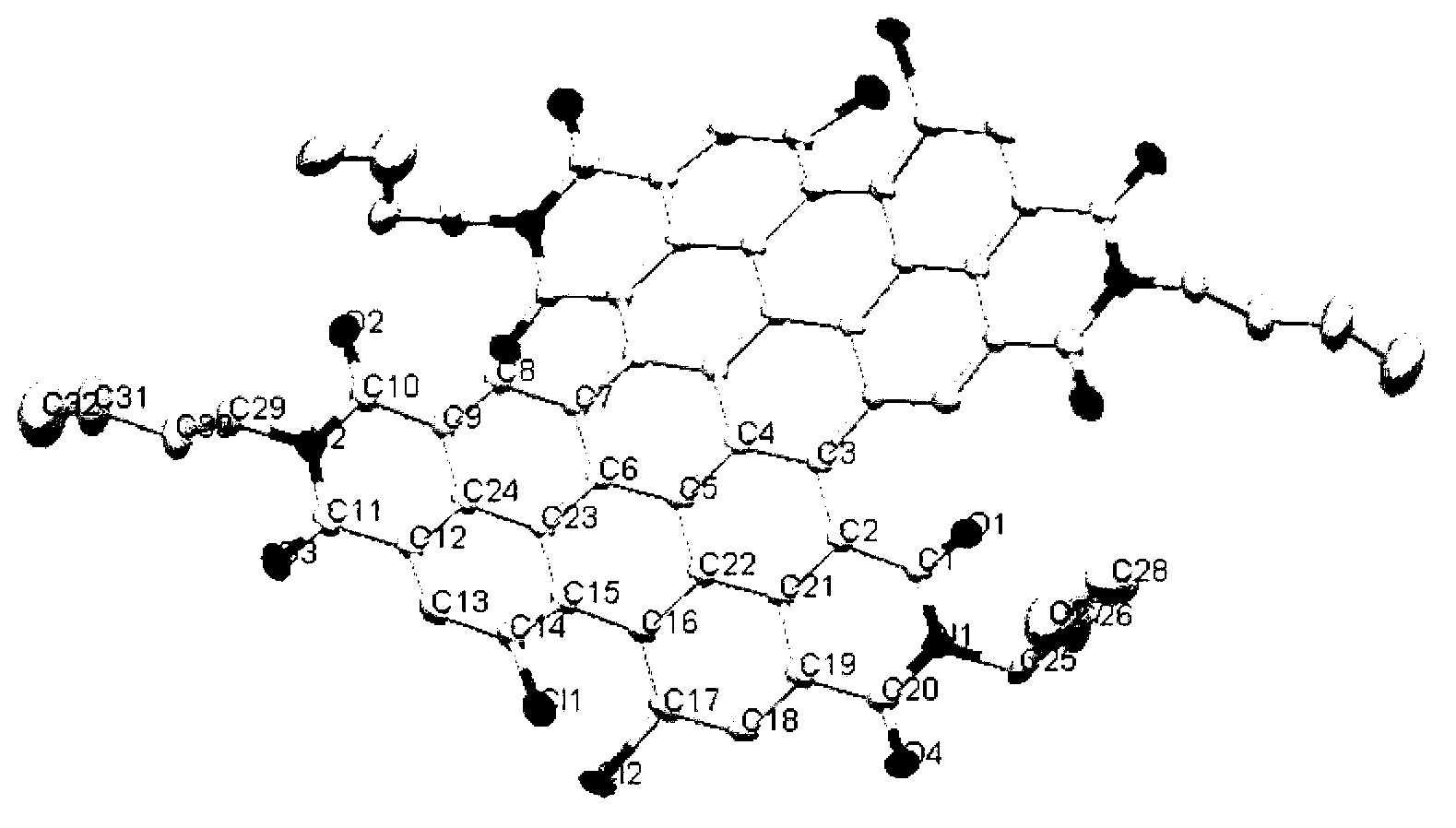
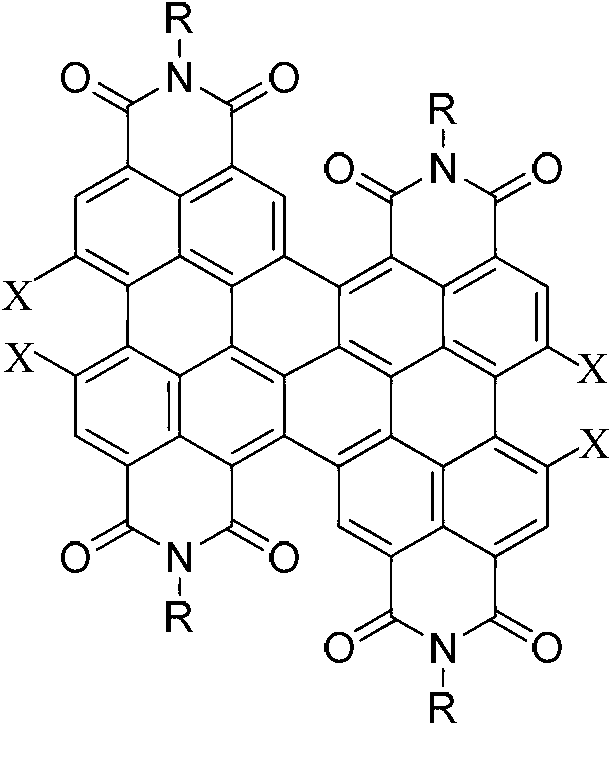
Method for preparing binary perylene diimide derivative efficiently
A compound and selected technology, applied in the direction of organic chemistry, can solve the problems of many by-products, long reaction time, environmental pollution materials, etc., achieve the effect of easy separation and purification, cheap raw materials, and expand the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, preparation C4-4CldiPBI (2a) (R=C in the formula I 4 h 9 , X=Cl compound)
[0033] In a 100mL two-necked round-bottom flask, evacuate and ventilate with argon. Then add tetrachloroperylene diimide raw material (1a) (R=C in the formula II 4 h 9 , X=Cl compound) (1.28g, 2.0mmol), cuprous iodide (0.95g, 5.0mmol), potassium thiophene-2-carboxylate (0.83g, 5.0mmol), potassium tert-butoxide (0.56g, 5.0 mmol). Dimethyl sulfoxide (40 mL) was injected at room temperature, and stirred at 90° C. for 12 h. Stop heating, cool naturally, pour the reaction mixture into 200mL deionized water, and stir well. Filter the above mixed solution with a Buchner funnel, and wash the filter cake with 100 mL of saturated ammonia water to remove a large amount of copper ions. The filter cake was washed with 300 mL of deionized water, 100 mL of anhydrous methanol, and dried at 100°C. Finally, the filter cake was dissolved in 100 mL of dichloromethane, mixed with diatomaceous e...
Embodiment 2
[0039] Embodiment 2, preparation C4-4CldiPBI (2a)
[0040] The preparation method was basically the same as in Example 1, except that the stirring at 90°C for 12 hours was replaced by stirring at 70°C for 15 hours in Example 1 to obtain 0.43 g of a purple-black solid (yield: 38%).
Embodiment 3
[0041] Embodiment 3, preparation C4-4CldiPBI (2a)
[0042]The preparation method was basically the same as that in Example 1, except that the stirring at 90°C for 12 hours in Example 1 was replaced by stirring at 120°C for 8 hours to obtain 0.35 g of a purple-black solid (yield: 31%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com