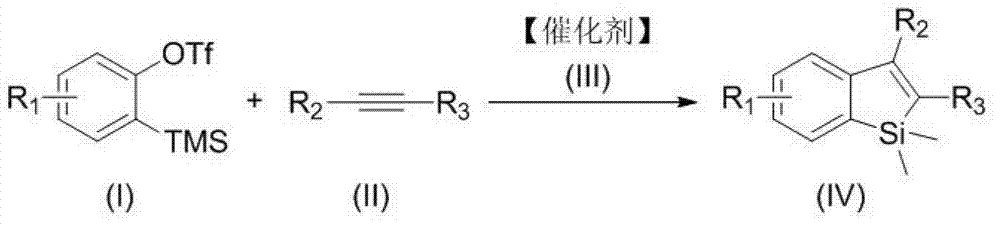
Synthesis method of polysubstituted silole derivative
A technology for the synthesis of silacyclopentadiene, applied in the direction of silicon organic compounds, etc., can solve the problems of inability to effectively realize multiple substituent groups, limit the diversity of substituent groups, and low tolerance of functional groups, so as to facilitate development The application and synthesis method are scientific and reasonable, and the effect of wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
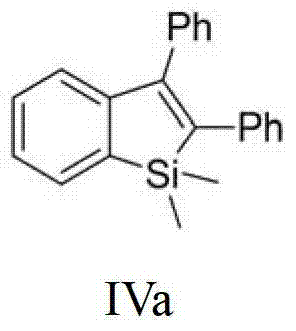
[0030] Embodiment 1——preparation formula IVa (R 1 =H,R 2 =R 3 =Ph) compound:
[0031]
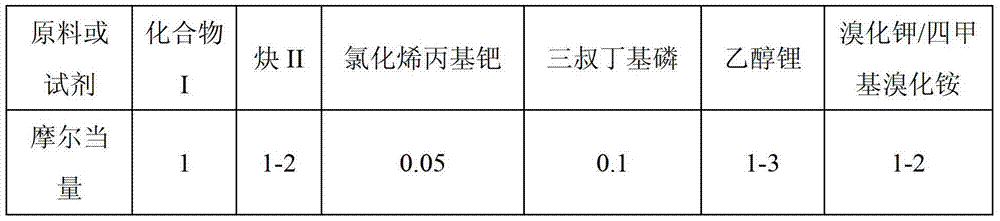
[0032] Under nitrogen protection, in a 50mL reaction tube, add 1mmol of 2-(trimethylsilyl)phenyltrifluoromethanesulfonate, 1.2mmol of tolan, 0.05mmol of allyl palladium chloride, 0.1mmol of Tri-tert-butylphosphine, 3 mmol of lithium ethoxide, 2 mmol of potassium bromide and 5 mL of toluene solvent. The reaction temperature was kept constant at 120° C. for 24 hours using an oil bath. After cooling and filtering, the filtrate was concentrated to obtain the crude product. The crude product was decolorized and separated on a silica gel column, and petroleum ether was used as the eluent to obtain 250 mg of 1,1-dimethyl-2,3-diphenylbenzosilole (purity>98%, colorless solid). The rate is 80%. The NMR data of this compound are as follows: 1 H NMR (400MHz, CDCl 3 )δ:0.47(s,6H,CH 3 ), 6.96-6.99 (m, 2H, CH), 7.04-7.07 (m, 2H, CH), 7.11-7.14 (m, 2H, CH), 7.18-7.21 (m, 2H, CH), 7.26-7.34 ( m...
Embodiment 2
[0033] Embodiment 2——preparation formula IVb (R 1 =OMe,R 2 =Ph,R 3 =TMS) compound:
[0034]
[0035] Under nitrogen protection, add 1mmol of 2-(trimethylsilyl)-3-methoxyphenyltrifluoromethanesulfonate, 1.2mmol of phenylethynyltrimethylsilane, 0.05mmol in a 50mL reaction tube Allylpalladium chloride, 0.1mmol of tri-tert-butylphosphine, 3mmol of lithium ethoxide, 2mmol of potassium bromide and 5mL of xylene solvent. The reaction temperature was kept constant at 120° C. for 24 hours using an oil bath. After cooling and filtering, the filtrate was concentrated to obtain the crude product. The crude product was decolorized and separated on a silica gel column, and petroleum ether was used as the eluent to obtain 220 mg of 1,1-dimethyl-2-(trimethylsilyl)-3-phenyl-7-methoxybenzosilole ( Purity>98%, colorless oily liquid), isolated yield 65%. The NMR data of this compound are as follows: 1 H NMR (400MHz, CDCl 3 )δ:0.49(s,6H,CH 3 ),3.85(s,3H,CH 3 ),6.69(d,J=7.5Hz,1H,CH),6....
Embodiment 3
[0036] Embodiment 3——preparation formula IVc (R 1 =2F,R 2 =R 3 =Ph) compound:
[0037]
[0038]Under nitrogen protection, add 1 mmol of 2-(trimethylsilyl)-4,5-difluorophenyltrifluoromethanesulfonate, 2 mmol of tolan, and 0.025 mmol of chlorinated alkenes into a 50 mL reaction tube Propyl palladium dimer, 0.1 mmol of tri-tert-butylphosphine, 2 mmol of lithium ethoxide, 1 mmol of tetramethylammonium bromide and 6 mL of 1,4-dioxane solvent. The reaction temperature was kept constant at 120° C. for 12 hours using an oil bath. After cooling and filtering, the filtrate was concentrated to obtain the crude product. The crude product was decolorized and separated on a silica gel column, and petroleum ether was used as the eluent to obtain 174 mg of 1,1-dimethyl-3,4-diphenyl-5,6-difluorobenzosilole (purity>98% , white solid), isolated in 50% yield. The NMR data of this compound are as follows: 1 H NMR(CDCl3,399.78MHz)δ:0.47(s,9H),6.85(dd,JH-F=11.8Hz,JH-F=7.6Hz),6.93-6.96(m,2H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com