Method for reversible chain transfer catalytic polymerization of polymerization system under phosphine catalysis
A catalytic polymerization and chain transfer technology, which is applied in the field of reversible chain transfer catalytic polymerization, can solve the problems of polymer performance impact, crosslinking, and wide molecular weight distribution index of polymers, and achieve the effect of efficient synthesis and convenient acquisition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
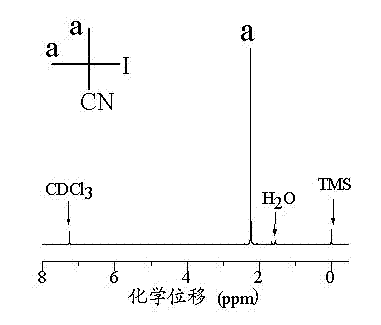
[0038] Step 1) Synthesis of iodoisobutyronitrile
[0039] see figure 1 As shown, weigh 25.0 mmol of azobisisobutyronitrile, measure about 50 mL of benzene and place it in a three-necked flask, and protect it with argon; weigh 50.4 mmol of iodine, add it to the above solution, and then add about 30 mL of benzene; Heat to 90 °C and reflux for 6-8 h; remove the reaction and cool to room temperature, add about 80 mL of saturated Na 2 S 2 o 3 solution, stirred until the color faded to light yellow; separatory funnel liquid separation, the lower liquid (aqueous solution) was discarded, and the upper layer (organic layer) was washed with saturated Na 2 S 2 o 3The solution (40 mL×2) was washed twice, and the upper layer (organic layer) was poured into the Erlenmeyer flask (the outer side of the Erlenmeyer flask was wrapped with aluminum foil), and about 15 scoops of anhydrous Na 2 SO 4 , placed on top of the refrigerator to dry overnight. Post-processing (vacuum distillation)...
Embodiment 2
[0053] Embodiment 2: Different iodine reagents take triphenylphosphine as a catalyst to prepare polymer PSt by reversible chain transfer catalytic polymerization
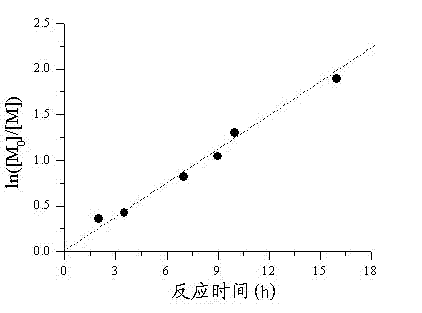
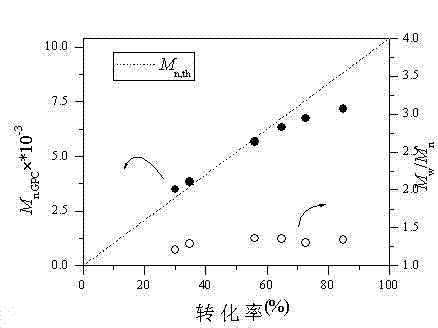
[0054] The difference with Example 1 is: the reaction ratio is [styrene] 0 : [Iodoisobutyronitrile] 0 : [Dicumyl Peroxide] 0 :[Triphenylphosphine] 0 = 100:0.1-1:0.1-1:0.1, the polymer PSt was obtained by reaction, and the results are shown in Table 2.
[0055] In Table 2, St is styrene, CPI is iodoisobutyronitrile, DCP is dicumyl peroxide, and TPP is triphenylphosphine, M n,th For the theoretically calculated molecular weight, M n,GPC and M w / M n Polymer molecular weight and molecular weight distribution determined for gel chromatography. GPC is gel chromatography. The data in row 1-4 in Table 2 is [styrene] 0 : [Dicumyl Peroxide] 0 =100: the polymerization result at 0.1-1; the data in rows 5-7 in Table 2 are the polymerization results of different amounts of iodoisobutyronitrile and different amou...
Embodiment 3
[0059] Example 3: Preparation of Polymer PSt by Reversible Chain Transfer Catalytic Polymerization under Different Phosphine Catalysts
[0060] The difference with Example 1 is: the reaction ratio is [styrene] 0 : [Iodoisobutyronitrile] 0 : [Dicumyl Peroxide] 0 : [P-Cata] 0 = 100:0.1-1:0.1-1:0.1, P-Cata in order: triphenylphosphine TPPO, TBPBr, CH 2 P 2 Ph 4 , C 2 h 4 P 2 Ph 4 One of them, the reaction yields polymer PSt with controllable activity.
[0061] In Table 3, TPPO is triphenylphosphine oxide, TBPBr is tetra-n-butylphosphine bromide, CH 2 P 2 Ph 4 is bis(diphenylphosphine)methane, C 2 h 4 P 2 Ph 4 is 1,2-bis(diphenylphosphine)ethane, M n,th For the theoretically calculated molecular weight, M n,GPC and M w / M n Polymer molecular weight and molecular weight distribution determined for gel chromatography. GPC is gel chromatography, and the data in rows 1-4 are the polymerization results of different phosphine catalysts. The results show that...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com