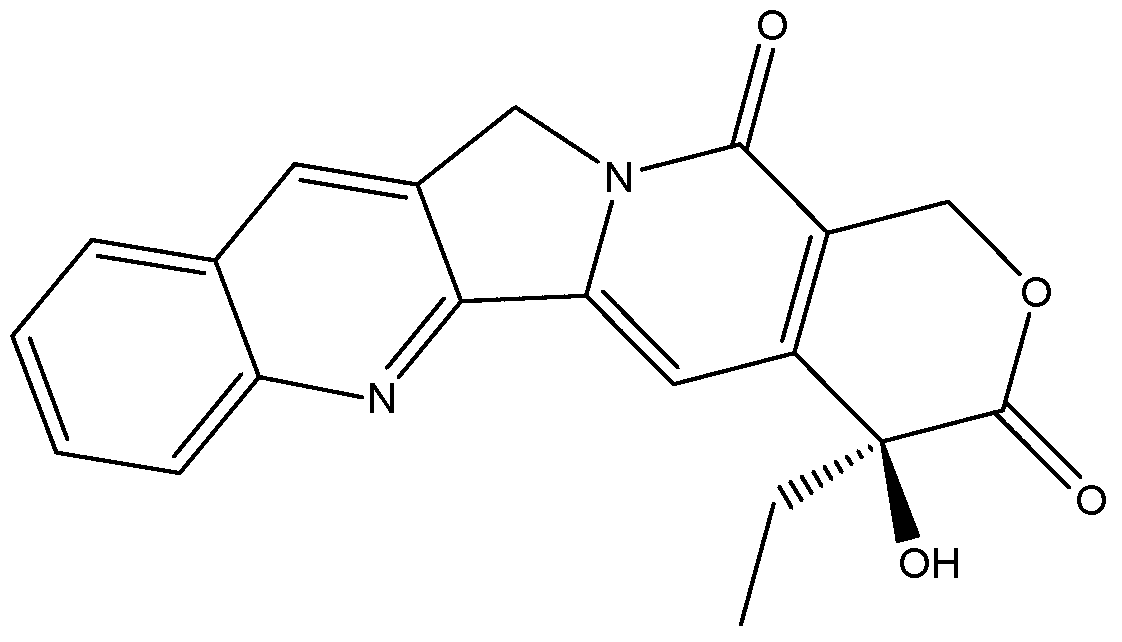
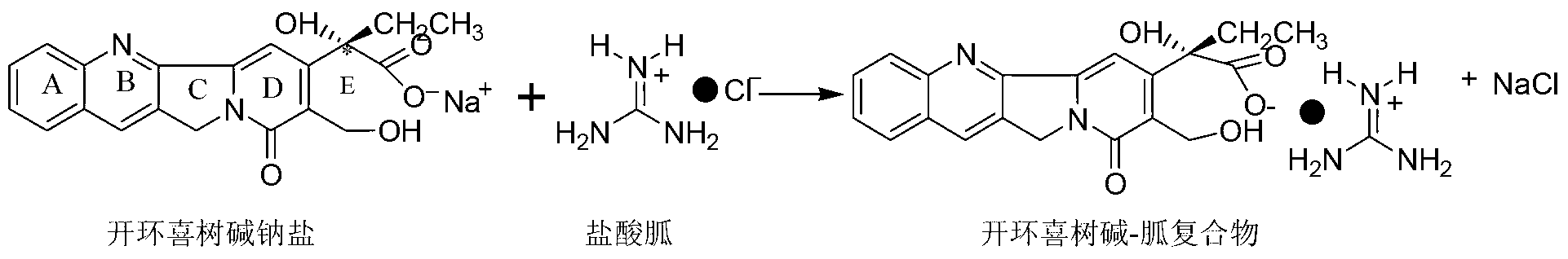
Separating and purifying method of camptothecin
A technology for separation and purification of camptothecin, applied in the direction of organic chemistry, which can solve the problems of long operation time, high production cost, complicated operation process, etc., and achieve the effect of simple operation, low cost and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In a 100ml three-neck flask equipped with a condensing reflux tube, add 8.75g of a commercially available camptothecin mixture (camptothecin content 80%, about 0.02mol), first use 20ml2mol / L sodium hydroxide solution to open the ring, and the sodium hydroxide The mol ratio with camptothecin is 2:1, then add 5.4g (about 0.03mol) guanidine carbonate respectively, the mol ratio of guanidine carbonate and camptothecin is 1.5:1, and the mixture of 60ml methanol and water (volume ratio is 2:1), at 65°C, stir and heat for 2.5 hours, stop the reaction, let it stand at -10°C for 1h after natural cooling, and precipitate the complex crystals of ring-opening camptothecin and separation reagent, and use 30ml of methanol-water mixed solvent (Volume ratio is 1:3) recrystallization, filtration, add the filter cake to 60ml5mol / L hydrochloric acid, the ring-opened camptothecin will be re-closed to obtain a camptothecin precipitate, and the camptothecin precipitate is obtained by filterin...
Embodiment 2
[0028] In a 100ml three-neck flask equipped with a condensing reflux tube, add 10g of a commercially available camptothecin mixture (camptothecin content 70%, about 0.02mol), first use 20ml2mol / L sodium hydroxide solution to open the ring, and then add 5.4 g (about 0.03mol) of guanidine carbonate, and 30ml of methanol and water mixture volume (volume ratio is 2:1), stirred and heated at 40°C for 3 hours, stopped the reaction, cooled naturally and left at 40°C for 10h, then precipitated and opened the ring The compound crystal of camptothecin and separation reagent is recrystallized with 30ml methanol and water mixed solvent (1:3 volume ratio), filtered, and the filter cake is added to 60ml5mol / L hydrochloric acid, and the ring-opened camptothecin The camptothecin precipitate was obtained by re-closing the ring, and the camptothecin precipitate was filtered, washed with water and dried to obtain 6.4 g of solid, with a yield of 91.4% and a purity of 98.9% by HPLC.
Embodiment 3
[0030] In a 100ml three-neck flask equipped with a condensing reflux tube, add 10.7g of a commercially available camptothecin mixture (camptothecin content 65%, about 0.02mol), first use 20ml2mol / L sodium hydroxide solution to open the ring, and then add 3.8g (about 0.04mol) guanidine hydrochloride, and 70ml of mixed solvent of ethanol and water (volume ratio: 1:1), stirred and heated at 80°C for 2 hours, stopped the reaction, cooled naturally and stood at 15°C for 4h, then precipitated Cyclocamptothecin and separation reagent complex crystals, recrystallized with 30ml methanol and water mixed solvent (volume ratio: 1:2), suction filtration, add the filter cake to 60ml5mol / L hydrochloric acid, the ring-opened camptothecin The ring was closed again to obtain camptothecin precipitate, which was filtered, washed and dried to obtain 6.5 g of solid, with a yield of 92.5% and a purity of 98.0% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com