Photochemical reaction synthesis for isoflavones
A technology of isoflavones and bromoisoflavones, applied in the field of heterocyclic compounds, can solve the problems of complex operation, complex reaction process, and use, and achieve the effects of mild reaction conditions, low production cost, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
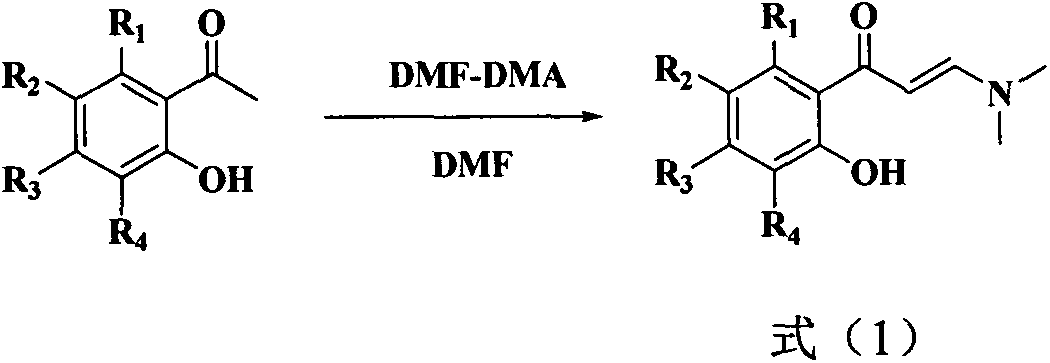
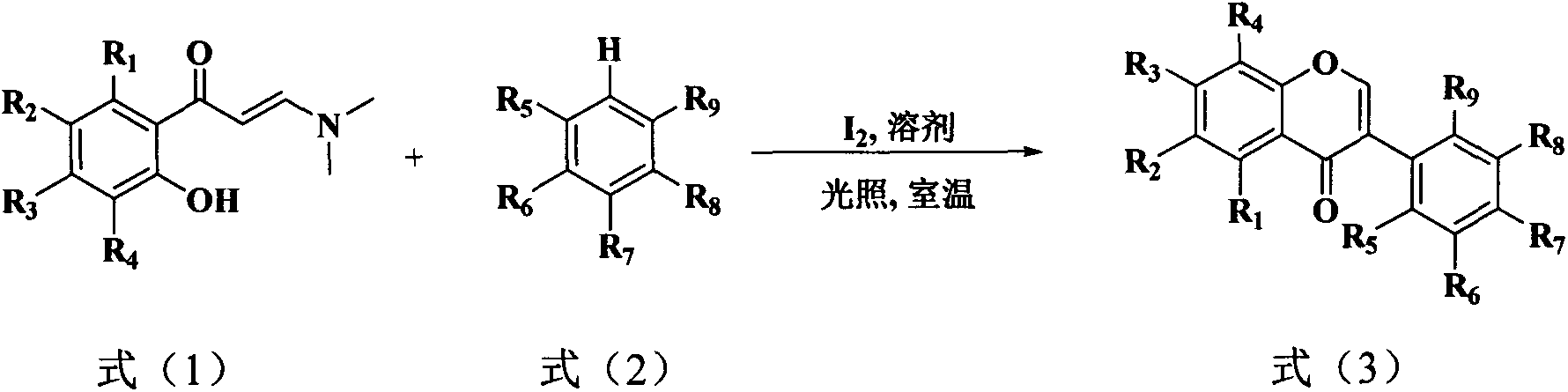
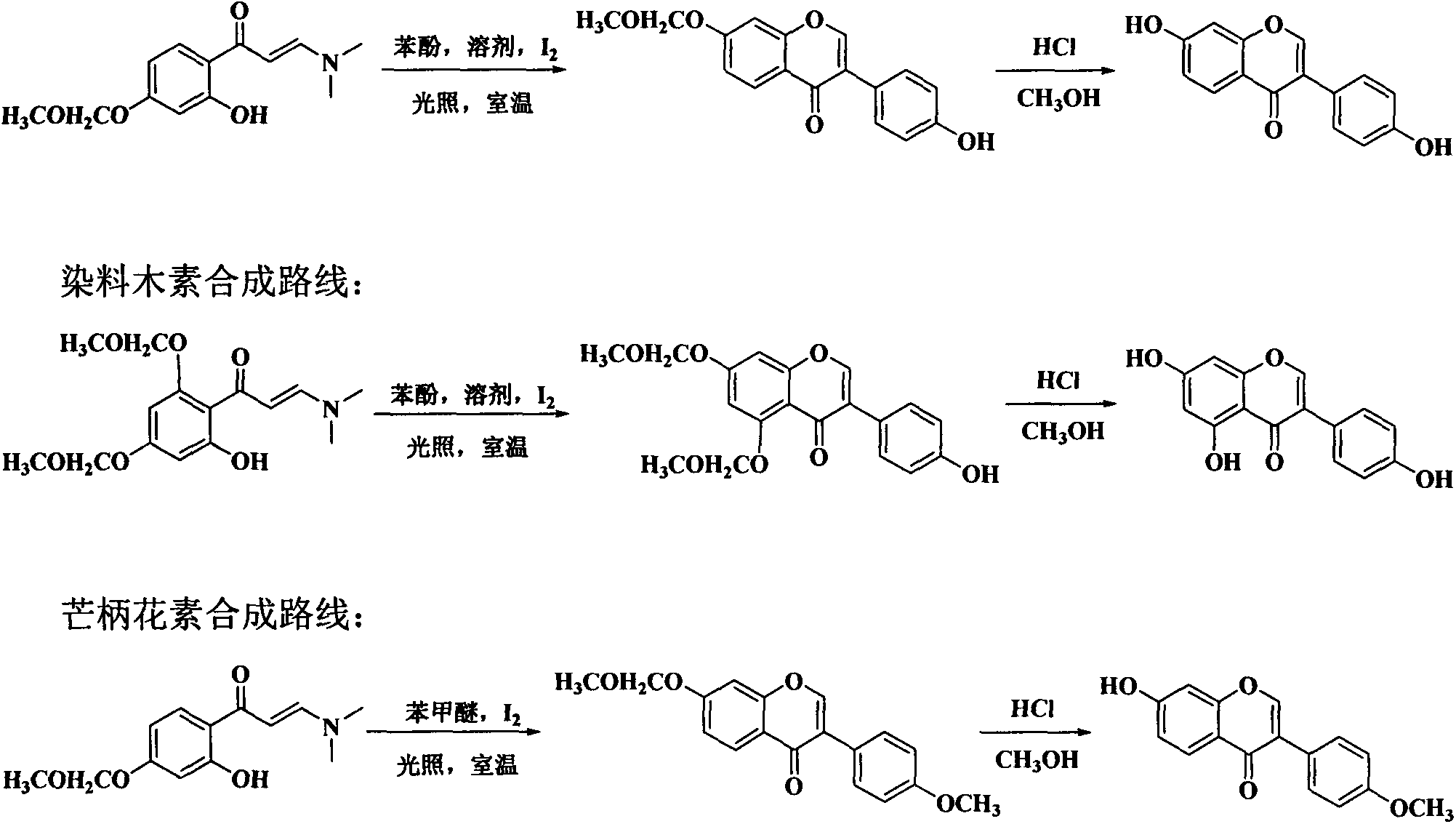
[0033] In this example, 3-(dimethylamino)-1-(2-hydroxyphenyl)prop-2-en-1-one, 3-(dimethylamino )-1-(2-hydroxy-4-methoxyphenyl)prop-2-en-1-one, 3-(dimethylamino)-1-(2-hydroxy-4-isopropoxyphenyl ) prop-2-en-1-one, 3-(dimethylamino)-1-(2-hydroxy-5-fluorophenyl) prop-2-en-1-one, 3-(dimethylamino)- 1-(2-hydroxy-5-chlorophenyl)prop-2-en-1-one, 3-(dimethylamino)-1-(2-hydroxy-5-bromophenyl)prop-2-en- 1-ketone and the benzene of formula (1) 310 times of weight, after stirring evenly, add the iodine simple substance of formula (1) 2 times of molar quantity, stop reaction after reacting under light for 10 hours, reclaim solvent by distillation under reduced pressure, use sherwood oil A mixed solvent with ethyl acetate volume ratio of 40:1 was used as eluent and separated by gradient elution of silica gel column chromatography to obtain compound (1) isoflavone, compound (2) 7-methoxy isoflavone, compound ( 3) 7-isopropoxyisoflavone (ipriflavone), compound (4) 6-fluoroisoflavone, compoun...
Embodiment 2
[0089] In this example, 3-(dimethylamino)-1-(2-hydroxyphenyl)prop-2-en-1-one, 3-(dimethylamino )-1-(2-hydroxy-4-methoxyphenyl)prop-2-en-1-one, 3-(dimethylamino)-1-(2-hydroxy-4-isopropoxyphenyl ) prop-2-en-1-one, 3-(dimethylamino)-1-(2-hydroxy-5-fluorophenyl) prop-2-en-1-one, 3-(dimethylamino)- 1-(2-hydroxy-5-chlorophenyl)prop-2-en-1-one, 3-(dimethylamino)-1-(2-hydroxy-5-bromophenyl)prop-2-en- 1-ketone and p-xylene of 350 times the weight of formula (1), after stirring evenly, add iodine elemental substance of formula (1) 2 times the molar weight, stop the reaction after reacting under light for 8 hours, the separation process and method of the product are the same as The preparation of the compound in Example 1 is the same, respectively to obtain compound (7) 2', 5'-dimethyl isoflavone, compound (8) 2', 5'-dimethyl-7-methoxy isoflavone, compound (9) 2′, 5′-dimethyl-7-isopropoxy isoflavone, compound (10) 2′, 5′-dimethyl-6-fluoroisoflavone, compound (11) 2′, 5 '-Dimethyl-6-ch...
Embodiment 3
[0145] In this example, 3-(dimethylamino)-1-(2-hydroxyphenyl)prop-2-en-1-one, 3-(dimethylamino )-1-(2-hydroxy-4-methoxyphenyl)prop-2-en-1-one, 3-(dimethylamino)-1-(2-hydroxy-4-isopropoxyphenyl ) prop-2-en-1-one, 3-(dimethylamino)-1-(2-hydroxy-5-fluorophenyl) prop-2-en-1-one, 3-(dimethylamino)- 1-(2-hydroxy-5-chlorophenyl)prop-2-en-1-one, 3-(dimethylamino)-1-(2-hydroxy-5-bromophenyl)prop-2-en- 1-ketone and the mesitylene of formula (1) 320 times weight, add the iodine simple substance of formula (1) 2 times molar weight again after stirring, stop reaction after reacting 6 hours under light, the separation process and method of product and The preparation of the compound in Example 1 is the same, respectively to obtain compound (13) 2', 4', 6'-trimethyl isoflavone, compound (14) 2', 4', 6'-trimethyl-7-form Oxygenated isoflavones, compound (15) 2', 4', 6'-trimethyl-7-isopropoxy isoflavone, compound (16) 2', 4', 6'-trimethyl-6- Fluoroisoflavones, compound (17) 2',4',6'-trimethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com