Substituted tricyclo-quinone compounds, and preparation method and application thereof
A technology for tricyclic quinones and compounds, which is used in the application of substituted tricyclic quinones and their preparation, as an antifungal drug, and can solve the problems of low bioavailability, large toxic and side effects, and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
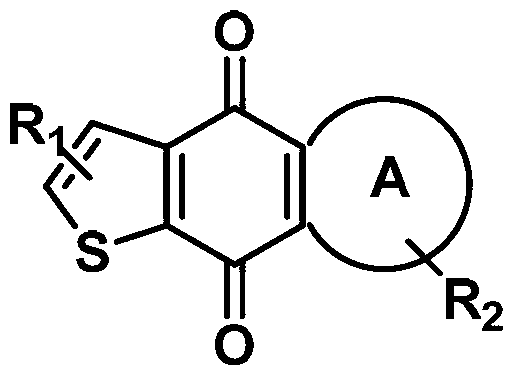
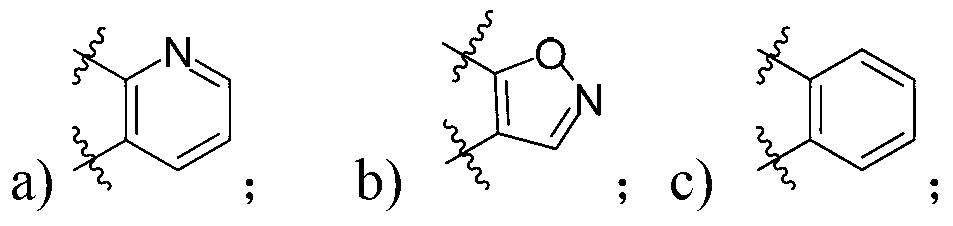
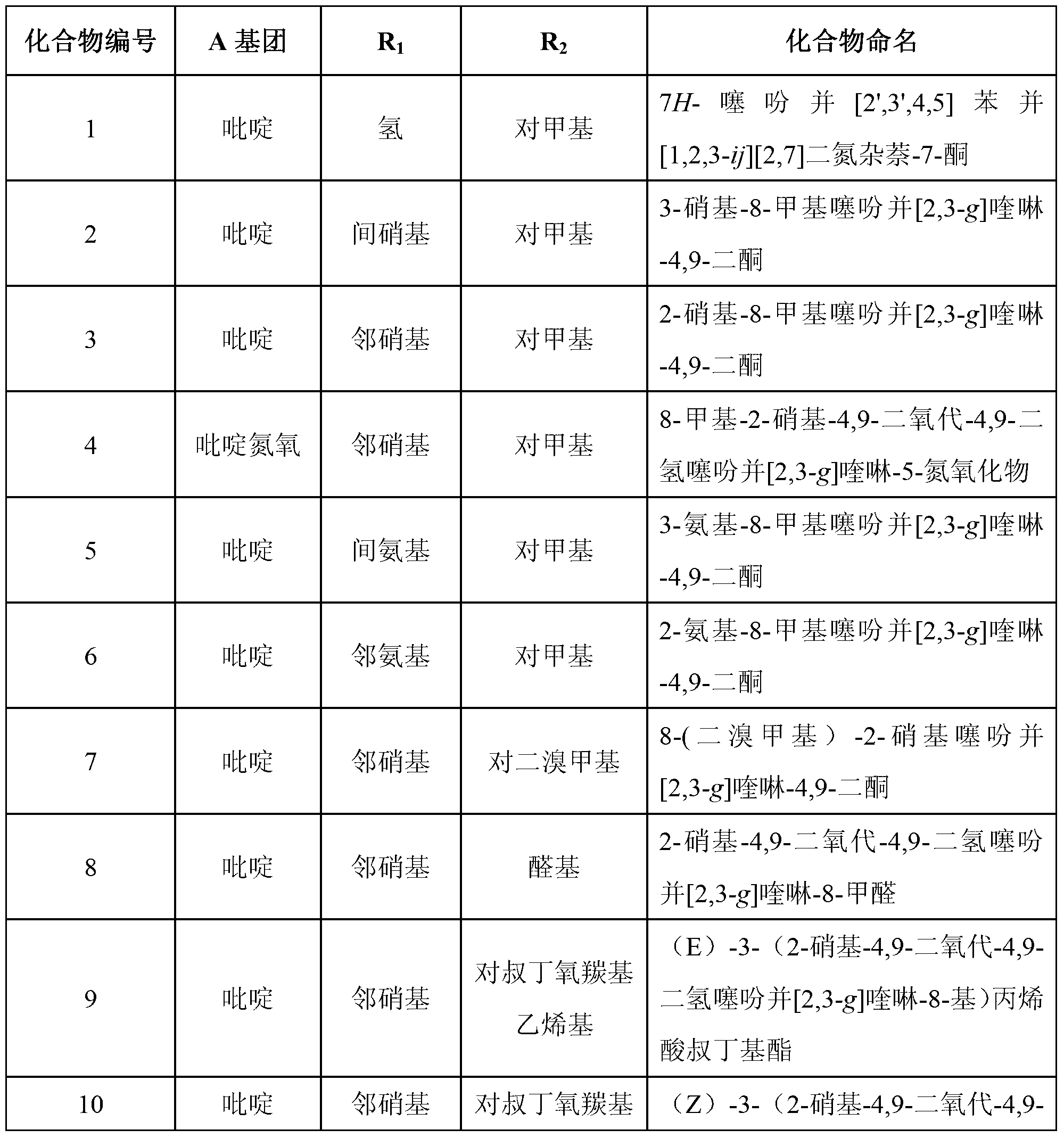
Image
Examples
Embodiment 1
[0114] Example 1: Preparation of 5,5-dibromo-6,7-dihydrobenzo[b]thiophene-4(5H)-one (II)
[0115] Add CuBr to a 500 mL round bottom flask 2 (28.2g, 126mmol, 4eq) and 80mL ethyl acetate. Heat this suspension to 80°C for a few minutes. Then 80 mL of a chloroform solution in which 6,7-dihydrobenzo[b]thiophene-4(5H)-one VIII (4.78 g, 31.45 mmol) was dissolved was added. After the addition was complete, the mixture was stirred overnight at this temperature. After the reaction of starting materials was complete the mixture was concentrated to dryness in vacuo. The residue was diluted with ethyl acetate and filtered through alumina. Filtrate with saturated NaHCO 3 After washing, the organic phase was dried over sodium sulfate and concentrated after filtration to obtain 9.5 g of white crystal product with a yield of 97%. 1 H NMR (400MHz, CDCl 3 )δppm: 7.51(d,J=5.2Hz,1H),7.20(d,J=5.2Hz,1H),3.18(s,4H).
Embodiment 2
[0116] Example 2: Preparation of 5-bromobenzo[b]thiophen-4-ol (Ⅲ)
[0117] The compound 5,5-dibromo-6,7-dihydrobenzo[b]thiophen-4(5H)-one (9.6 g, 31 mmol) and DMF (100 mL) were added into a 250 mL round bottom flask. To this solution was added lithium carbonate (14 g, 186. mmol, 6 eq). The reaction mixture was heated to 100°C under nitrogen for 6 hours. The reaction solution was then cooled to room temperature and filtered. The filtrate was diluted with water, acidified to pH = 1 with HCl, and extracted with ethyl acetate. The organic phase was washed with water and brine, concentrated and purified by column chromatography (eluent: ethyl acetate: petroleum ether = 1:50) to obtain 6.82 g of white solid with a yield of 95.8%. 1 H NMR (400MHz, CDCl 3 )δppm:7.52(d,J=5.2Hz,1H),7.41(d,J=5.2Hz,1H),7.40(d,J=8.4,1H),7.35(d,J=8.4,1H),5.89 (br s,4H).
Embodiment 3
[0118] Example 3: Preparation of 5-bromobenzo[b]thiophene-4,7-dione (IV)
[0119] Into a 500 mL round bottom flask was added phenol 5-bromobenzo[b]thiophen-4-ol (4.6 g, 20 mmol), 80 mL acetic acid, 120 mL trifluoroacetic acid and a few drops of water. The reaction mixture was cooled to zero with an ice-water bath, and then iodobenzene diacetate (19.3 g, 60 mmol, 3 eq) was added in portions. After the addition was complete, the mixture was stirred for an additional 10 minutes and at room temperature for 20 minutes. Then 100 mL of methanol was added, and after stirring for 10 minutes, water and dichloromethane were added. The organic phase was separated and concentrated. The concentrated residue was purified by silica gel column chromatography (eluent: ethyl acetate:petroleum ether=1:50) to obtain 3.91 g of a yellow solid with a yield of 80.5%. 1 H NMR (400MHz, CDCl 3 )δppm: 7.73(d, J=5.2Hz, 1H), 7.66(d, J=5.2Hz, 1H), 7.42(s, 1H); 13 C NMR (100MHz, CDCl 3 )δppm: 177,174,14...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com