Method for generating lactam by acetylpropionic acid conversion
A technology of levulinic acid and lactam, which is applied in the direction of organic chemistry, can solve the problems of limited scope of application of substrates, unstable phosphine ligands, and high requirements for reactors, and achieve low production costs, simple operation, and high reaction yields Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
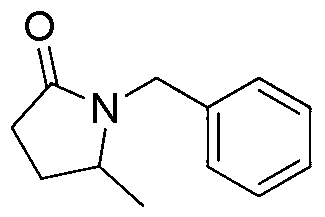
[0015] Preparation of 1-benzyl-5-methyl-2-pyrrolidone with the following structural formula
[0016]
[0017] Under argon protection, add 116mg (1mmol, 0.1mL) levulinic acid, 214mg (2mmol) benzylamine, 230mg (5mmol) formic acid, 101mg (1mmol) triethylamine, 3mL dimethyl sulfoxide into thick-walled pressure-resistant tube Add a magnet to stir, react at 100°C for 4 hours, cool to room temperature, adjust the pH value to alkaline with a saturated aqueous solution of sodium hydroxide, extract with dichloromethane (5×3mL), and dry the organic phase with anhydrous sodium sulfate. Distilled under reduced pressure to remove dichloromethane, use a mixture of petroleum ether and ethyl acetate with a volume ratio of 3:1 (triethylamine is added to the mixture, and the amount added is 1% of the volume of the mixture) as eluent , the product was separated by flash column chromatography and prepared into 1-benzyl-5-methyl-2-pyrrolidone with a yield of 87%. The characterization data of the...
Embodiment 2
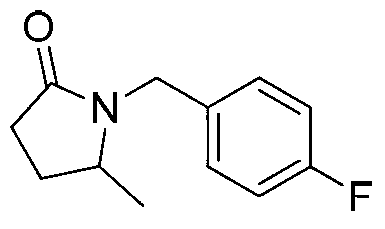
[0019] Preparation of 1-(4-fluoro)-benzyl-5-methyl-2-pyrrolidone of the following structural formula
[0020]
[0021] Under argon protection, add 116mg (1mmol) levulinic acid, 250mg (2mmol) 4-methylbenzylamine, 230mg (5mmol) formic acid, 101mg (1mmol) triethylamine, 3mL dimethyl sulfoxide In the tube, add a magnet to stir, react at 100°C for 12 hours, cool to room temperature, and other steps are the same as in Example 1 to prepare 1-(4-fluoro)-benzyl-5-methyl-2-pyrrolidone, which produces The rate is 89%, and the characterization data of the product are: 1 H NMR (400MHz, CDCl 3)δ(ppm):7.22-7.19(m,2H),6.99(t,J=8.6Hz,2H),4.87(d,J=15.0Hz,1H),3.98(d,J=15.0Hz,1H) ,3.51(sextet,J=6.2Hz,1H),2.53-2.34(m,2H),2.19-2.10(m,1H),1.64-1.55(m,1H),1.15(d,J=6.2Hz,3H ); 13 C NMR (100MHz, CDCl 3 )δ (ppm): 175.0, 162.2 (d, 1 J C-F =244.1Hz), 132.7(d, 4 J C-F =3.7Hz), 129.6(d, 3 J C-F =8.0Hz), 115.5(d, 2 J C-F =21.4Hz), 53.0, 43.3, 30.2, 26.7, 19.6; HRMS(ESI)C 12 h 14 FNO[M+Na] ...
Embodiment 3
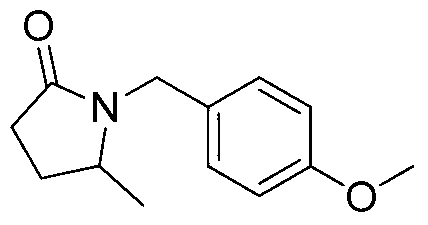
[0023] Preparation of 1-(4-methoxy)-benzyl-5-methyl-2-pyrrolidone of the following structural formula
[0024]
[0025] Under argon protection, add 116mg (1mmol) levulinic acid, 246mg (2mmol) 4-methylbenzylamine, 230mg (5mmol) formic acid, 101mg (1mmol) triethylamine, 3mL dimethyl sulfoxide In the tube, add a magnet to stir, react at 100°C for 12 hours, and cool to room temperature. The other steps are the same as in Example 1 to prepare 1-(4-methoxy)-benzyl-5-methyl-2-pyrrolidone. Its productive rate is 89%, and the characteristic data of product are: 1 H NMR (400MHz, CDCl 3 )δ(ppm):7.13(d,J=8.6Hz,2H),6.81(t,J=8.6Hz,2H),4.87(d,J=14.8Hz,1H),3.99(d,J=14.8Hz ,1H),3.75(s,3H),3.48(sextet,J=6.2Hz,1H),2.50-2.31(m,2H),2.15-2.06(m,1H),1.59-1.50(m,1H), 1.13(d,J=6.2Hz,3H); 13 C NMR (100MHz, CDCl 3 )δ (ppm): 174.9, 159.0, 129.3, 128.8, 114.0, 55.2, 52.7, 43.3, 30.2, 26.6, 19.6; HRMS (ESI) C 13 h 17 NO 2 [M+Na] + : The theoretical value is 242.1157, and the experimental value ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com