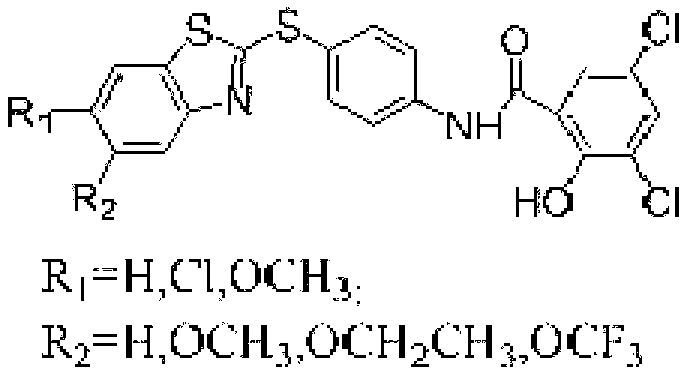Preparation method of micromolecule cathepsin D inhibitor
A technology of small molecules and inhibitors, which is applied in the field of preparation of small molecule cathepsin D inhibitors, can solve the problems of long reaction time, low yield, pollution and the like, and achieves the effects of simple operation, simple reaction conditions and simple post-treatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
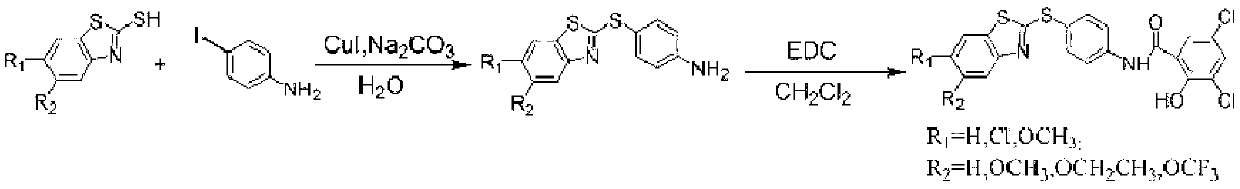
[0021] (I) Preparation of 4-(2-benzothiazylthio)aniline
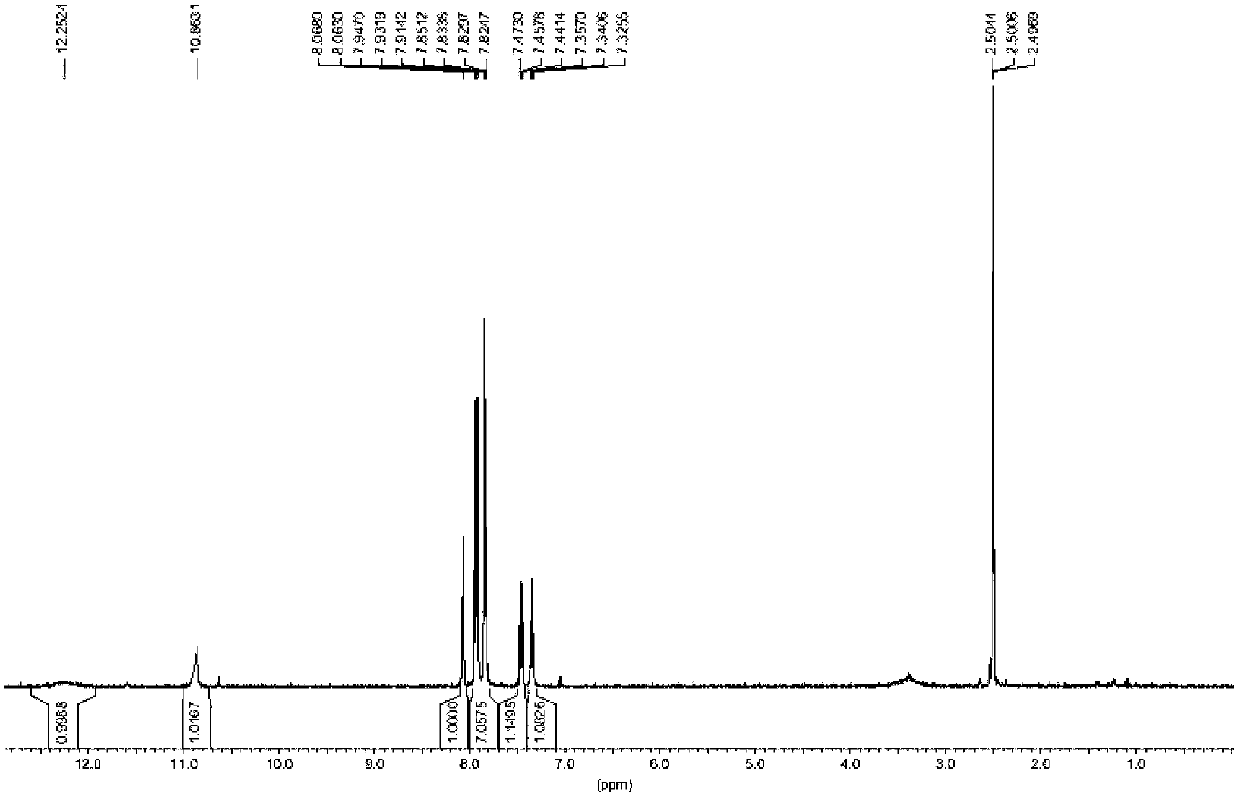
[0022] First add 2-mercaptobenzothiazole (1.67g, 10mmol), p-aminoiodobenzene (2.63g, 12mmol), catalyst CuI (0.19g, 1mmol), Na 2 CO 3 (1.06g, 10mmol) and H 2 O (20mL), heated to 120°C and refluxed for 24h, the reaction was completed, cooled to room temperature, the reaction liquid was extracted with ethyl acetate, the obtained organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and spin-dried, PE:EA= 2.09 g of 4-(2-benzothiazylsulfanyl)aniline was obtained by passing through the column at 6:1, with a yield of 86.0%.
[0023] (Π) Preparation of a small molecule cathepsin D inhibitor
[0024] Dissolve 4-(2-benzothiazylthio)aniline (1.29g, 5mmol) and 3,5-dichloro-2-hydroxybenzoic acid (1.04g, 5mmol) in CH 2 CL 2 (20mL), add the acylating agent 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) (0.96g, 5mmol), heat to 40°C under reflux for 8h, After the reaction is complet...
Embodiment 2
[0026] (I) Preparation of 4-(2-(6-chlorobenzothiazylthio))aniline
[0027] First add 2-mercapto-6-chlorobenzothiazole (2.02g, 10mmol), p-aminoiodobenzene (2.63g, 12mmol), catalyst CuI (0.19g, 1mmol), Na 2 CO 3 (1.06g, 10mmol) and H 2 O (20mL), heated to 120°C and refluxed for 24h, the reaction was completed, cooled to room temperature, the reaction liquid was extracted with ethyl acetate, the obtained organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and spin-dried, PE:EA= 2.46 g of 4-(2-(6-chlorobenzothiazylsulfanyl))aniline was obtained in a 6:1 column with a yield of 84.0%.
[0028] (Π) Preparation of a small molecule cathepsin D inhibitor
[0029] Dissolve 4-(2-(6-chlorobenzothiazylthio))aniline (1.46g, 5mmol) and 3,5-dichloro-2-hydroxybenzoic acid (1.04g, 5mmol) in CH 2 CL 2 (20mL), add the acylating agent 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) (0.96g, 5mmol), heat to 40°C under reflux for 8h, After the re...
Embodiment 3
[0031] (I) Preparation of 4-(2-(6-methoxybenzothiazylthio))aniline
[0032] First add 2-mercapto-6-methoxybenzothiazole (1.97g, 10mmol), p-aminoiodobenzene (2.63g, 12mmol), catalyst CuI (0.19g, 1mmol), Na 2 CO 3 (1.06g, 10mmol) and H 2 O (20mL), heated to 120°C and refluxed for 24h, the reaction was completed, cooled to room temperature, the reaction liquid was extracted with ethyl acetate, the obtained organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and spin-dried, PE:EA= 2.39 g of 4-(2-(6-methoxybenzothiazylsulfanyl))aniline was obtained by 6:1 column, with a yield of 83.2%.
[0033] (Π) Preparation of a small molecule cathepsin D inhibitor
[0034] 4-(2-(6-methoxybenzothiazylthio))aniline (1.44g, 5mmol) obtained in step 1 was dissolved in 3,5-dichloro-2-hydroxybenzoic acid (1.04g, 5mmol) in CH 2 CL 2 (20mL), add the acylating agent 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) (0.96g, 5mmol), heat to 40°C under r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com