Novel method for producing dabigatran etexilate
A technology for dabigatran etexilate and products, which is applied in the new field of dabigatran etexilate production, can solve the problems of unsuitability for scale-up production, high cost, poor selectivity, etc., and achieves that raw materials and reagents are cheap and easy to obtain, and the cost is low. , the effect of high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
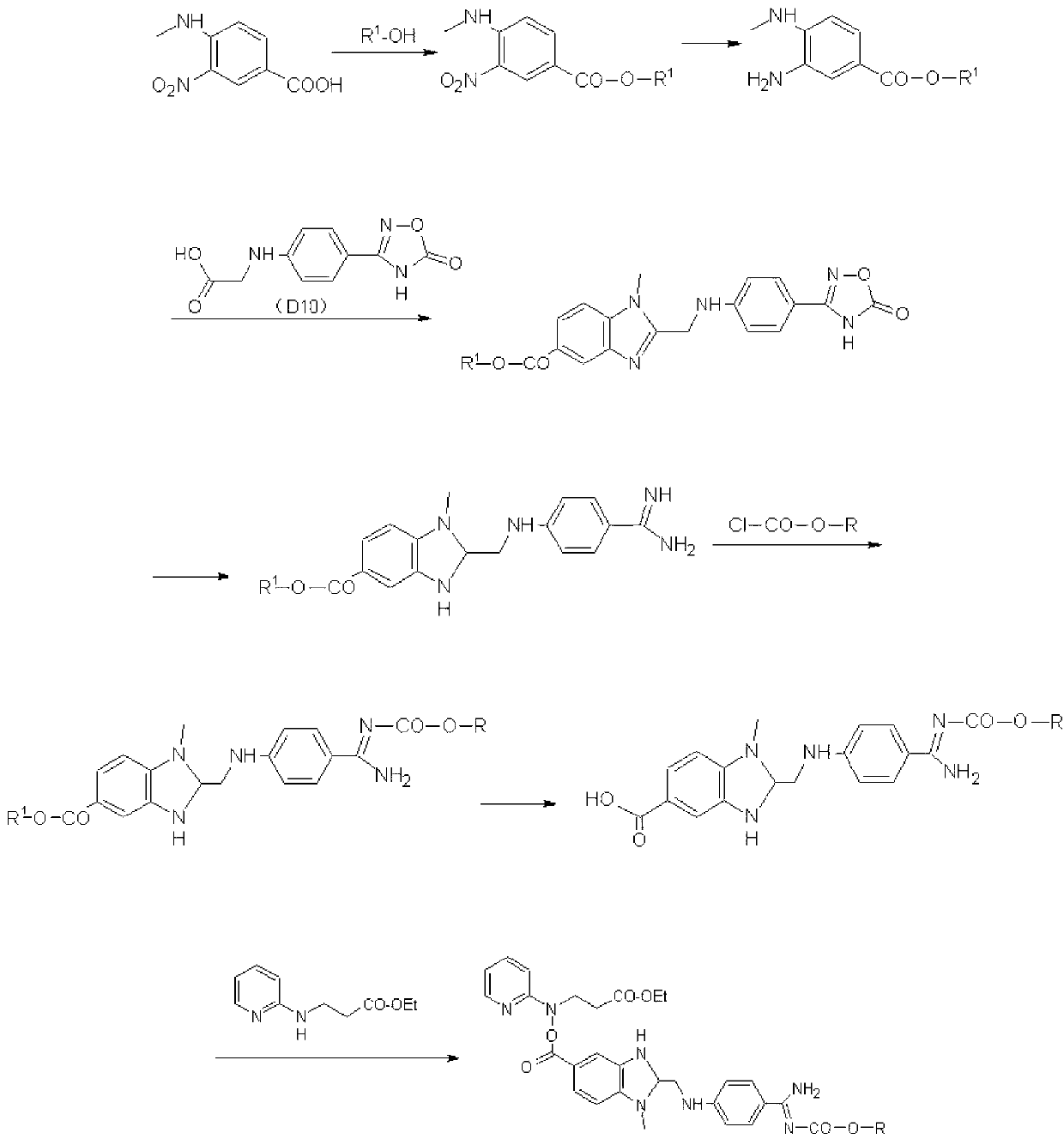
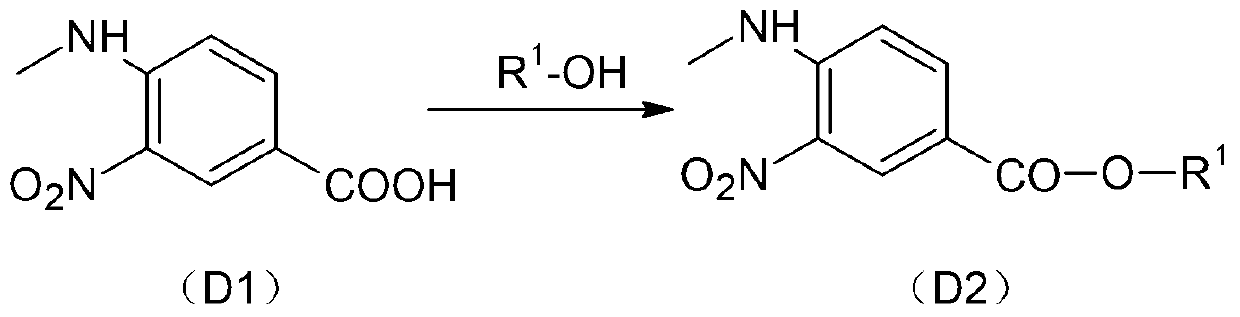
[0080] Step 1): The reaction is carried out in a 500ml four-neck reaction flask, under the protection of preferably an inert gas, 60g (0.306mol) of D1 (4-methylamino-3-nitro-benzoic acid, CAS: 41263-74 -5, or refer to: Zhang Weiguang et al., Synthesis of 3-(2-pyridylamino) ethyl propionate process research, Synthetic Chemistry, 2012 No. 69) and 300ml of toluene in the catalyst N,N-dimethylformaldehyde Amide (catalytic amount: 2 drops) was incubated at 90°C and reacted at 90°C. When the system temperature was 60°C, 73g (0.613mol) of thionyl chloride was added dropwise. After the system was clarified, it reacted for about 1 hour and then concentrated and then added 300ml of ethanol , Add 32g (0.316mol) triethylamine dropwise when the temperature is raised to 60-65°C, and keep the reaction for 1 hour. Concentrate the reaction solution to dryness, dissolve the concentrate in dichloromethane, wash with saturated sodium bicarbonate solution (100ml) and water (100ml) successively, an...
Embodiment 2
[0088] Step 1): Under the protection of nitrogen, react 0.25mol of D1 with 300ml of toluene in the catalyst N,N-dimethylformamide (catalytic amount: 2 drops) at 88°C, wherein, when the system temperature is 56°C , add 0.501 mol of thionyl chloride dropwise, react for about 1 hour after system clarification, add 300 ml of ethanol after concentration, add 0.253 mol of triethylamine dropwise at 60-65°C, and keep warm for 1 hour. Concentrate the reaction solution to dryness, dissolve the concentrate in dichloromethane, wash with saturated sodium bicarbonate solution and water successively, dry and dehydrate, crystallize, and filter to obtain yellow crystal D2; the yellow crystal D2 requires a purity of >98.5%, and the The rate is 87.3%.
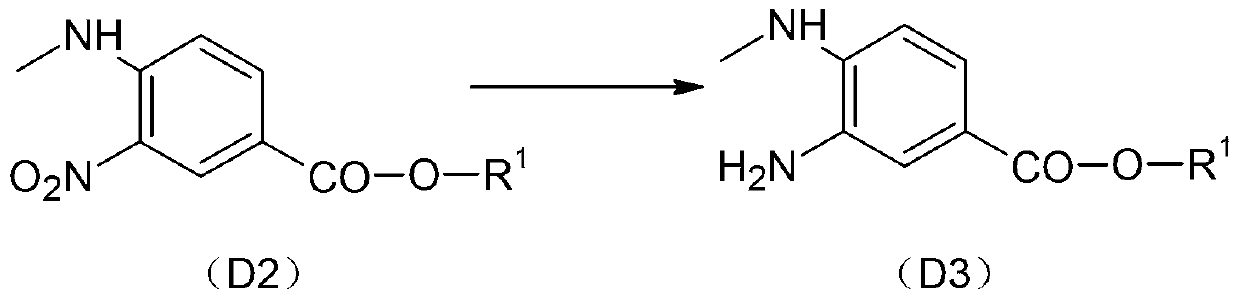
[0089] Step 2): Warm up 0.25mol of D2 and 300ml of ethyl acetate to 60°C, heat up to 60°C, after the system is clarified, put in 10% palladium carbon; replace the air for hydrogenation, after 2 hours of reaction, cool down Below 40°C, crystalliz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com