Chromatographic purification method of fatty acid mono-acylation insulin
A chromatographic purification and insulin technology, which is applied in the field of chromatographic purification, can solve the problems of easy damage to the chemical properties of reversed-phase silica gel, affect the separation effect of fillers, troublesome maintenance, etc., and achieve good separation and purification effects, good cost advantages, and simple steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] (1) Preparation of samples to be purified:
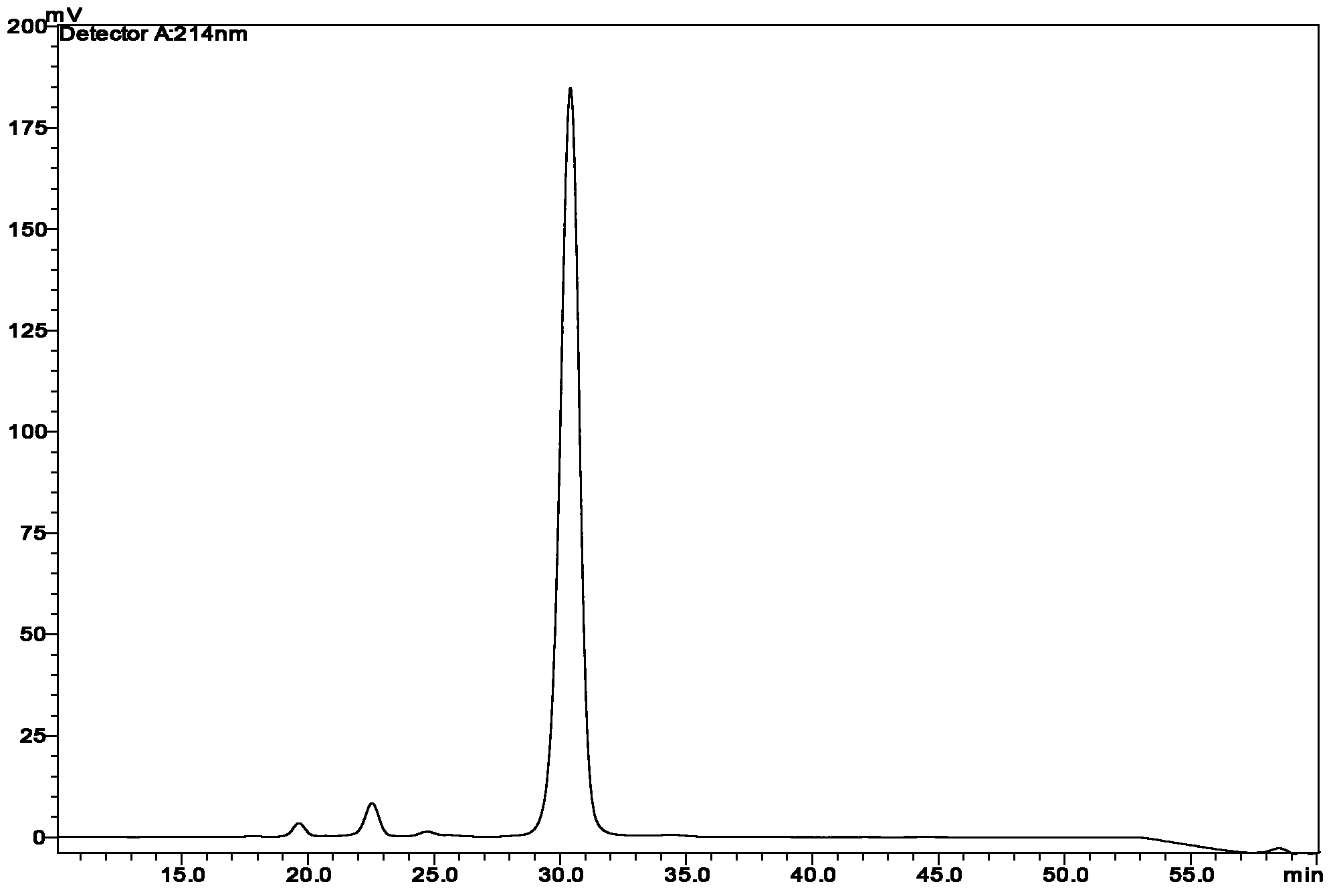
[0056] Referring to Example 7 disclosed in patent WO98 / 02460, 0.50 g of Des B30 human proinsulin was dissolved in a mixed solution of 7.0 ml of dimethylsulfoxide (DMSO) and 3.5 ml of water, and 0.4 ml of triethylamine was added thereto Adjust the pH to 10.2-10.5 to obtain a mixed solution. Then dissolve 0.025g of N-succinimidyl myristic acid in 1.8ml of N-methylpyrrolidone solution, and add it dropwise to the aforementioned mixed solution, and finally place the homogeneously mixed solution at 0°C and stir for 2 hours to react , you can get insulin derivatives containing acylation modification, namely (N εB29 -tetradecanoyl) Lys B29 des (B30) reaction solution of human insulin. Then it was acidified and diluted 10 times with an aqueous solution containing acetic acid (the concentration of acetic acid is 10% by volume) to obtain the sample to be purified. The purity of the sample to be purified is as Figure 16 shown.
[...
Embodiment 2
[0067] (1) Preparation of the sample to be purified: same as step (1) in Example 1.
[0068] (2) Purification using QuickSep medium pressure chromatography system:
[0069] A chromatographic column with a diameter of 11 mm and a length of 250 mm is filled with 10 ml of nano-micro Uni PS30-300 filler (particle size 30 μm, pore size 300 angstroms), and the bed height is 110 mm.
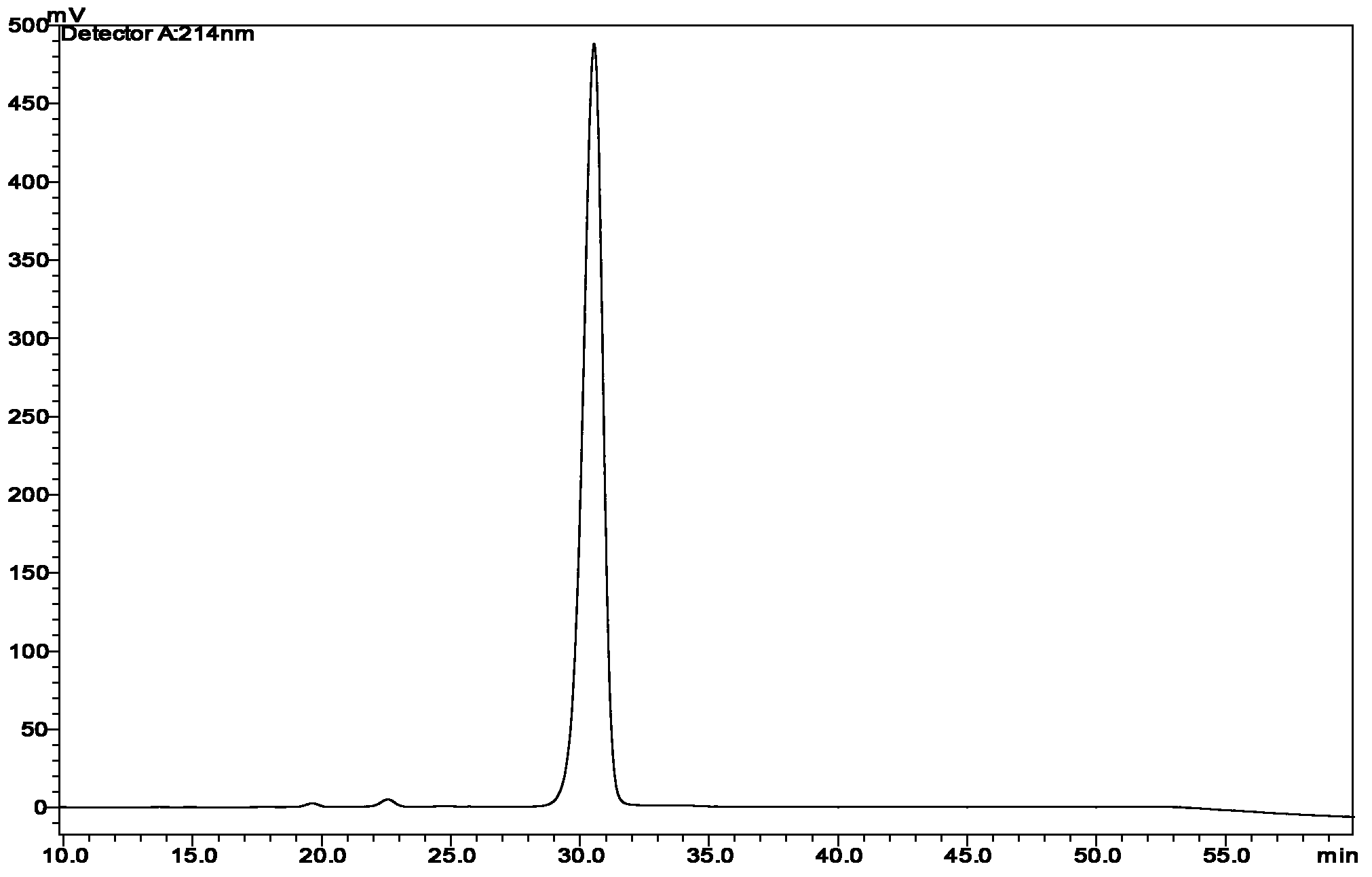
[0070] The mobile phase preparation ratio is the same as in Example 1, and the pH of the mobile phase A is adjusted to be 3.0. Adjust the amount of loaded sample so that the loading of the target protein in the column is 4.0 g / L. Adjust the operating pressure of the chromatography system to be 2 bar, 5 bar and 8 bar respectively. The elution method is the same as that in Example 1. After the peak is detected by a 280nm UV detector, it is collected in sections, each section is 10ml, and then the components with a purity greater than 98.0% are mixed, then the purity and concentration are detected, and t...
Embodiment 3
[0078] (1) Preparation of the sample to be purified: same as step (1) in Example 1.
[0079] (2) Purification using QuickSep medium pressure chromatography system:
[0080] A chromatographic column with a diameter of 140 mm and a length of 500 mm is filled with 2.5 L of GE source30 filler (particle size 30 μm), and the bed length is 165 mm.
[0081] The chromatography buffer solution is: phase A, 0.15M sodium acetate buffer solution, pH 3.0; phase B, acetonitrile solution containing TFA (trifluoroacetic acid), the concentration of TFA is 0.1% by volume.
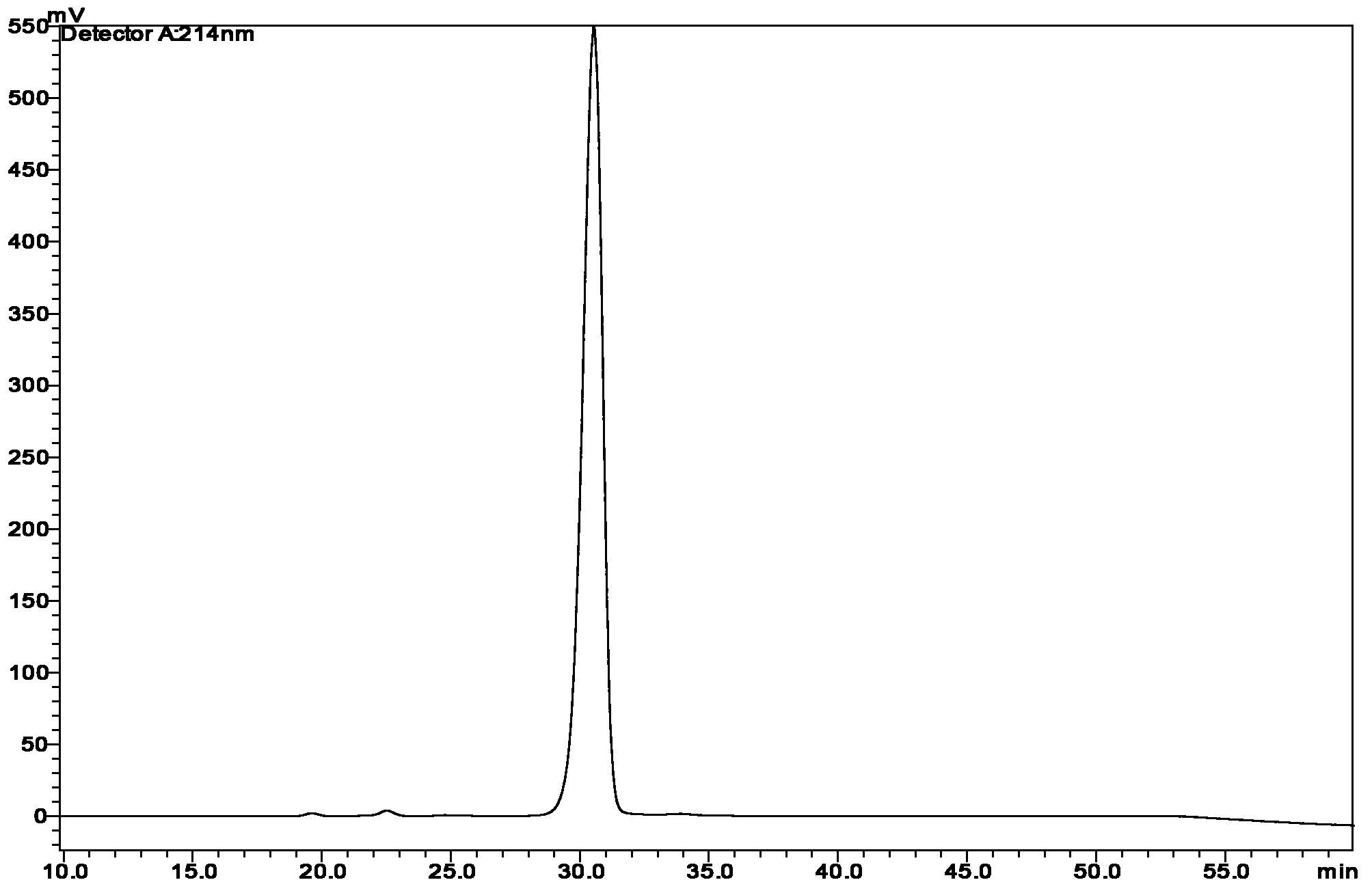
[0082] The column is first equilibrated with 10% B phase, that is, A phase and B phase are mixed at a volume ratio of 9:1. After equilibration, the sample is loaded so that the target protein load of the filler is 4.0g / L, and 40% B is eluted at an equal degree. The speed is 5.7L / h, the working pressure is 8bar, and the 280nm UV detector monitors the peak and collects it in sections, each section has a volume of 1.0L.
[008...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com