Polar liquid chromatogram filler and preparation method thereof
A high-performance liquid chromatography, polar technology, applied in the field of polar liquid chromatography packing and its preparation, can solve the problems of poor reproducibility of the two-step synthesis method, poor acid and alkali resistance of the packing, and poor batch reproducibility, etc. Excellent selectivity and resolution, strong separation ability, effect of eliminating influence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthesis of embodiment 1 silane and polar chromatography stationary phase
[0029] 1.1 Preparation of undecylglycine
[0030]
[0031] Add CH to a 250 mL three-necked flask 2 Cl 2 (50 mL), undecyl chloride (4.49 g) and triethylamine (5 mL), vigorously stirred in an ice bath, and CH containing glycine (1.5 g) was added dropwise 2 Cl 2 solution (20 ml). After the dropwise addition was completed, the reaction was stirred at room temperature for 2 hours. The crude product was purified by column chromatography using ethyl acetate / petroleum ether (3:1) as the eluent to obtain a colorless oily liquid product (4.53 g). 1 HNMR (500 MHz, CDCl 3 ) δ 0.84 (t, 3H), 1.29 (m, 14H), 1.58 (m, 2H), 2.15 (m, 2H), 4.16 (s, 2H). Calc. C% 64.16, H% 10.36, N% 5.76 ; Found C% 64.10, H% 10.38, N% 5.71.
[0032] 1.2 Preparation of silanes containing double amide bonds
[0033]
[0034] Undecylglycine (2.4 g) was added to the three-necked flask, N,N'- Dicyclohexylcarbodiim...
Embodiment 2
[0041] The following series of polar high performance liquid chromatography fillers containing double amide bonds were synthesized by a synthesis method similar to that of Example 1.
[0042]
[0043] The structure of the above-mentioned synthesized substance was confirmed by infrared, NMR and elemental analysis.
Embodiment 3
[0044] Example 3 Chromatographic column performance evaluation
[0045] 3.1 Packing of analytical column
[0046] The filler prepared in Example 1 is filled in a 150 x 4.6 mm I.D. stainless steel column tube by the homogenization method, and the filling pressure is preferably 40-80 MPa. The resulting column is used to separate mixture samples.
[0047] 3.2 Engelhardt test
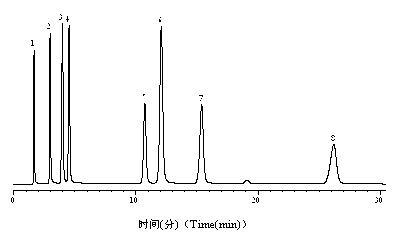
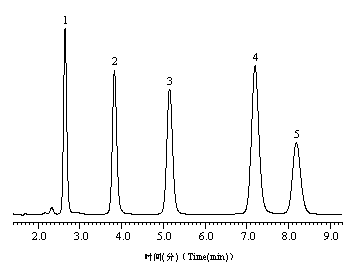
[0048] With the chromatographic column prepared in embodiment 3.1, 1 thiourea, 2 aniline, 3 o-, m-, p - Toluidine, 4 phenol, 5 N,N - Dimethylaniline, 6 ethyl benzoate, 7 toluene, 8 ethylbenzene mixture, figure 1 for its chromatogram. The chromatographic conditions are as follows: mobile phase, methanol:water=55:45 (v / v); flow rate, 1 mL / min; column temperature, 25 0 C; Detection wavelength, UV 254 nm.
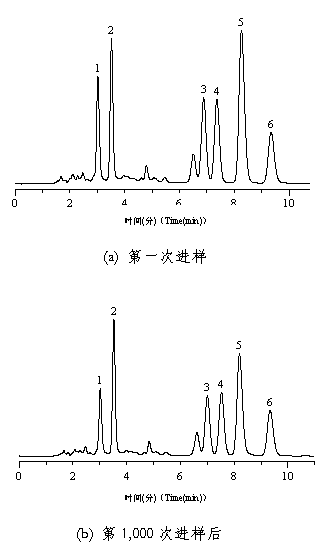
[0049] 3.3 Stability test
[0050] With the chromatographic column prepared in Example 3.1, the stability of the chromatographic column of the present invention at different pHs was measured ( ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com