Preparation method of anion exchange membrane based on biimidazole cation cross-linking agent
A technology of anion exchange membrane and ion cross-linking agent, which is applied to fuel cell parts, fuel cells, electrical components, etc., can solve the problems of poor chemical stability and achieve the effect of strong polymerization ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Biimidazolium cationic crosslinking agent Preparation of:
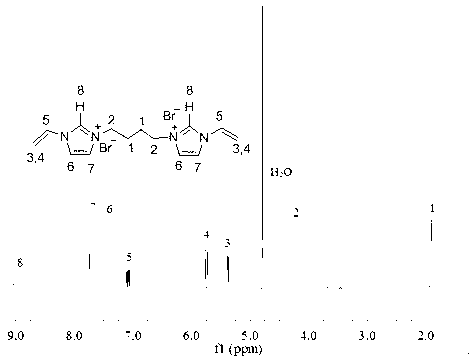
[0027] Under nitrogen protection, add 5.20g (0.055 mol) 1-vinylimidazole and 5.40g (0.025 mol) 1,4-dibromobutane to a three-necked flask equipped with 40ml of acetonitrile; Evaporate to obtain crude product, crude product is washed 3 times with ethyl acetate, vacuum-dried at room temperature 24 hours; Obtain final product 9.10g (productive rate 90.1%), use 1 H NMR. To characterize its structure, such as figure 1 Shown; The cross-linking agent of other structures used in the present invention is prepared with reference to this method.
[0028]
Embodiment 2
[0030] Acrylonitrile 0.10g, 0.20g, 0.20g, 0.01g, mixed evenly and applied to the mold, UV light (wavelength 240nm-380nm) for 30min, in-situ polymerization to form a film; then soak the anion exchange membrane in 60℃ 1M KOH solution for 24 hours to convert anions into OH - ; The OH obtained in this embodiment - The ion conductivity of the type anion exchange membrane at room temperature is 2.1×10 -2 S cm -1 , the ionic conductivity at 90°C is 5.98×10 -2 S cm -1 . The tensile strength is 9.12 Mpa, the Young's modulus is 476.29Mpa, and the elongation at break is 44.21%.
Embodiment 3
[0032] Acrylonitrile 0.20g, 0.20g, 0.20g, benzoin ethyl ether 0.01g, mix the solution evenly, apply it on the mold, irradiate with ultraviolet light (wavelength 240nm-380nm) for 30min, and polymerize in situ to form a film; then soak the anion exchange membrane in 60℃ 1M KOH solution for 24 hours , converting the anion to OH - ; The OH obtained in this embodiment - The ion conductivity of the type anion exchange membrane at room temperature is 1.66×10 -2 S cm -1 , the ionic conductivity at 90°C is 4.25×10 -2 S cm -1 , the tensile strength is 15.66 Mpa, the Young's modulus is 809.33Mpa, and the elongation at break is 32.08%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com