Preparation method of colloidal solution of nano-ZnO
A nano-zinc oxide and colloidal solution technology, applied in the direction of zinc oxide/zinc hydroxide, nanotechnology, nanotechnology, etc., can solve the problems of easy agglomeration, difficult control of particle size by chemical methods, large size dispersion, etc., and achieve particle size Uniformity, good biocompatibility and biodegradability, viscosity reducing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
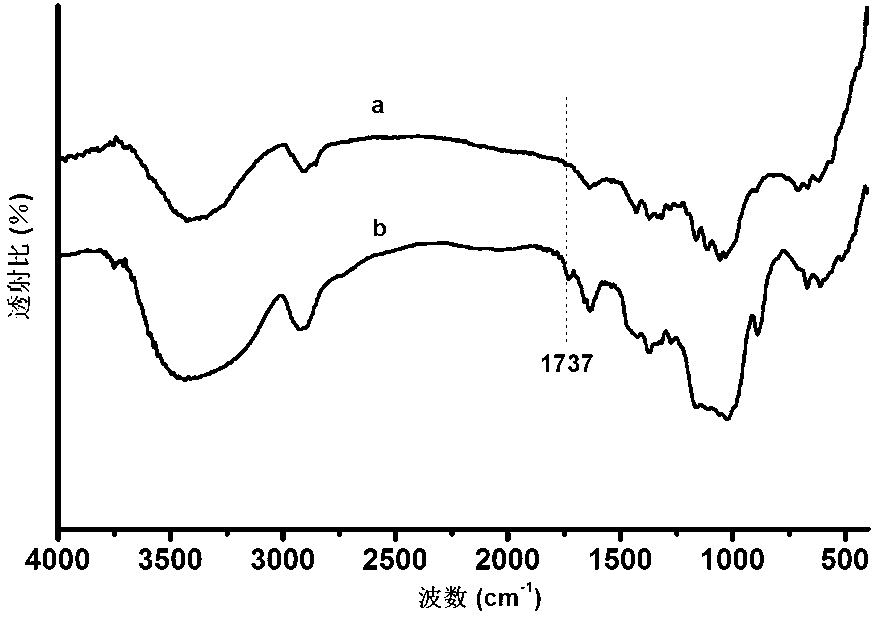
[0021] 1. Prepare dialdehyde hydroxypropyl cellulose according to the technical scheme provided in the literature "Selectively Oxidized Cotton Fiber Aggregate Structure". The preparation method is: first dissolve hydroxypropyl methylcellulose in deionized water to obtain the mass The percentage is 1% hydroxypropyl methylcellulose solution, and then the mass fraction of 1% sodium periodate aqueous solution is gradually dropped into the above solution, and the volume ratio of the two is 1:1 to 50:1. The reaction temperature was set at 50°C, and the reaction was fully stirred by mechanical stirring in a light-proof environment. After 30 minutes of reaction, the reaction solution was cooled to room temperature. Then, the above solution was dialyzed for 24 hours by using a dialysis bag with a dialysis molecular weight of 5000 to obtain pure dialdehyde hydroxypropyl methylcellulose. See attached figure 1 , it is the infrared spectrogram of the hydroxypropyl methyl cellulose before ...
Embodiment 2
[0029] 1. Prepare dialdehyde hydroxypropyl methylcellulose according to the technical scheme of embodiment 1. Dissolve 0.20 g of dialdehyde hydroxypropyl methylcellulose in 100 mL of deionized water to obtain an aqueous solution of dialdehyde hydroxypropyl methylcellulose with a concentration of 2 g / L;
[0030] 2. Dissolve 0.727g of zinc nitrate in 100mL of deionized water to obtain a zinc nitrate solution with a concentration of 7.27g / L;
[0031] 3. Dissolve 5g of sodium hydroxide in 100mL of deionized water to obtain an aqueous solution of sodium hydroxide with a concentration of 50g / L;
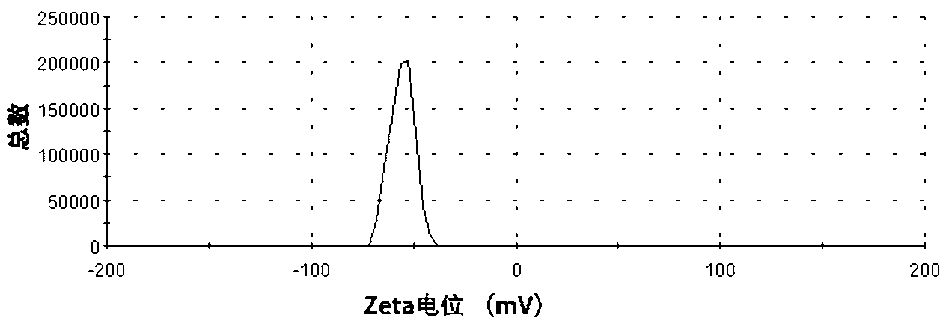
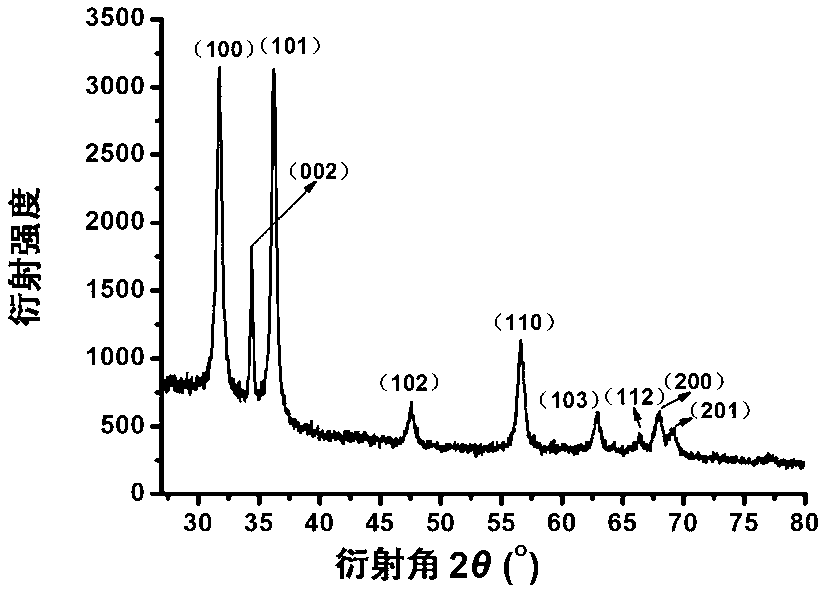
[0032]4. Mix 2g / L dialdehyde hydroxypropyl methylcellulose aqueous solution and zinc nitrate aqueous solution with a mass fraction of 7.87g / L at a volume ratio of 4:1, and add 50g / L solution dropwise under constant stirring at 70°C. L of sodium hydroxide aqueous solution (mass ratio of zinc nitrate to sodium hydroxide is 189:80), react for 2 hours to obtain a nano-zinc oxide colloidal solu...
Embodiment 3
[0034] 1. Dissolve 0.20 g of dialdehyde hydroxypropyl methylcellulose in 100 mL of deionized water to obtain a dialdehyde hydroxypropyl methylcellulose aqueous solution with a concentration of 2 g / L;
[0035] 2. Dissolve 0.727g of zinc nitrate in 100mL of deionized water to obtain a zinc nitrate solution with a concentration of 7.27g / L;
[0036] 3. Dissolve 5mL of ammonia water in 95mL of deionized water to obtain an aqueous ammonia solution;
[0037] 4. Mix 2g / L dialdehyde hydroxypropyl methylcellulose aqueous solution and zinc nitrate aqueous solution with a mass fraction of 7.87g / L at a volume ratio of 4:1, and add ammonia aqueous solution dropwise under constant stirring at 70°C (The mass ratio of zinc nitrate to ammonia water is 189:20). After 2 hours of reaction, a nano-zinc oxide colloidal solution with a particle size of 10-60nm can be obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com